Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry
- PMID: 23283320
- PMCID: PMC3711516
- DOI: 10.1523/JNEUROSCI.0245-12.2013
Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry
Abstract
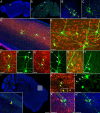
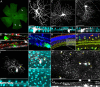
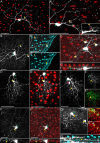
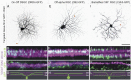
The use of neurotropic viruses as transsynaptic tracers was first described in the 1960s, but only recently have such viruses gained popularity as a method for labeling neural circuits. The development of retrograde monosynaptic tracing vectors has enabled visualization of the presynaptic sources onto defined sets of postsynaptic neurons. Here, we describe the first application of a novel viral tracer, based on vesicular stomatitis virus (VSV), which directs retrograde transsynaptic viral spread between defined cell types. We use this virus in the mouse retina to show connectivity between starburst amacrine cells (SACs) and their known synaptic partners, direction-selective retinal ganglion cells, as well as to discover previously unknown connectivity between SACs and other retinal ganglion cell types. These novel connections were confirmed using physiological recordings. VSV transsynaptic tracing enables cell type-specific dissection of neural circuitry and can reveal synaptic relationships among neurons that are otherwise obscured due to the complexity and density of neuropil.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
