Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats
- PMID: 23283321
- PMCID: PMC6618644
- DOI: 10.1523/JNEUROSCI.2422-12.2013
Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats
Abstract
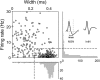
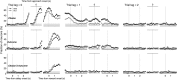
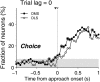
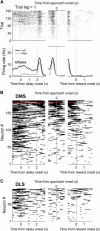
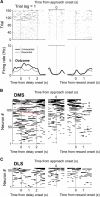
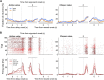
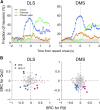
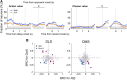
The cortico-basal ganglia network has been proposed to consist of parallel loops serving distinct functions. However, it is still uncertain how the content of processed information varies across different loops and how it is related to the functions of each loop. We investigated this issue by comparing neuronal activity in the dorsolateral (sensorimotor) and dorsomedial (associative) striatum, which have been linked to habitual and goal-directed action selection, respectively, in rats performing a dynamic foraging task. Both regions conveyed significant neural signals for the animal's goal choice and its outcome. Moreover, both regions conveyed similar levels of neural signals for action value before the animal's goal choice and chosen value after the outcome of the animal's choice was revealed. However, a striking difference was found in the persistence of neural signals for the animal's chosen action. Signals for the animal's goal choice persisted in the dorsomedial striatum until the outcome of the animal's next goal choice was revealed, whereas they dissipated rapidly in the dorsolateral striatum. These persistent choice signals might be used for causally linking temporally discontiguous responses and their outcomes in the dorsomedial striatum, thereby contributing to its role in goal-directed action selection.
Figures

Similar articles
-
Role of striatum in updating values of chosen actions.J Neurosci. 2009 Nov 25;29(47):14701-12. doi: 10.1523/JNEUROSCI.2728-09.2009. J Neurosci. 2009. PMID: 19940165 Free PMC article.
-
Neuronal activity in dorsomedial and dorsolateral striatum under the requirement for temporal credit assignment.Sci Rep. 2016 Jun 1;6:27056. doi: 10.1038/srep27056. Sci Rep. 2016. PMID: 27245401 Free PMC article.
-
Differential encoding of action selection by orbitofrontal and striatal population dynamics.J Neurophysiol. 2020 Aug 1;124(2):634-644. doi: 10.1152/jn.00316.2020. Epub 2020 Jul 29. J Neurophysiol. 2020. PMID: 32727312 Free PMC article.
-
Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action.Neuropsychopharmacology. 2010 Jan;35(1):48-69. doi: 10.1038/npp.2009.131. Neuropsychopharmacology. 2010. PMID: 19776734 Free PMC article. Review.
-
Cortico-basal ganglia networks subserving goal-directed behavior mediated by conditional visuo-goal association.Front Neural Circuits. 2013 Oct 21;7:158. doi: 10.3389/fncir.2013.00158. eCollection 2013. Front Neural Circuits. 2013. PMID: 24155692 Free PMC article. Review.
Cited by
-
Modeling Parkinson's disease falls associated with brainstem cholinergic systems decline.Behav Neurosci. 2015 Apr;129(2):96-104. doi: 10.1037/bne0000048. Behav Neurosci. 2015. PMID: 25798629 Free PMC article.
-
Stable Representations of Decision Variables for Flexible Behavior.Neuron. 2019 Sep 4;103(5):922-933.e7. doi: 10.1016/j.neuron.2019.06.001. Epub 2019 Jul 4. Neuron. 2019. PMID: 31280924 Free PMC article.
-
Hierarchical gradients of multiple timescales in the mammalian forebrain.Proc Natl Acad Sci U S A. 2024 Dec 17;121(51):e2415695121. doi: 10.1073/pnas.2415695121. Epub 2024 Dec 13. Proc Natl Acad Sci U S A. 2024. PMID: 39671181 Free PMC article.
-
Whether or not to act is determined by distinct signals from motor thalamus and orbitofrontal cortex to secondary motor cortex.Nat Commun. 2025 Apr 4;16(1):3106. doi: 10.1038/s41467-025-58272-w. Nat Commun. 2025. PMID: 40185746 Free PMC article.
-
A novel strategy for dissecting goal-directed action and arousal components of motivated behavior with a progressive hold-down task.Behav Neurosci. 2015 Jun;129(3):269-80. doi: 10.1037/bne0000060. Behav Neurosci. 2015. PMID: 26030428 Free PMC article.
References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. - PubMed
-
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
