Functional differentiation of a population of electrically coupled heterogeneous elements in a microcircuit
- PMID: 23283325
- PMCID: PMC3680137
- DOI: 10.1523/JNEUROSCI.3841-12.2013
Functional differentiation of a population of electrically coupled heterogeneous elements in a microcircuit
Erratum in
- J Neurosci. 2013 Feb 6;33(6):2728. Sasaki, Kosai [corrected to Sasaki, Kosei]
Abstract
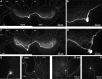
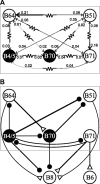
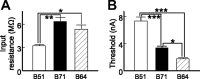
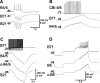
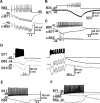
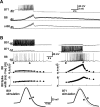
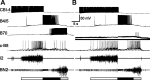
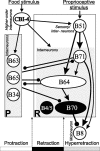
Although electrical coupling is present in many microcircuits, the extent to which it will determine neuronal firing patterns and network activity remains poorly understood. This is particularly true when the coupling is present in a population of heterogeneous, or intrinsically distinct, circuit elements. We examine this question in the Aplysia californica feeding motor network in five electrically coupled identified cells, B64, B4/5, B70, B51, and a newly identified interneuron B71. These neurons exhibit distinct activity patterns during the radula retraction phase of motor programs. In a subset of motor programs, retraction can be flexibly extended by adding a phase of network activity (hyper-retraction). This is manifested most prominently as an additional burst in the radula closure motoneuron B8. Two neurons that excite B8 (B51 and B71) and one that inhibits it (B70) are active during hyper-retraction. Consistent with their near synchronous firing, B51 and B71 showed one of the strongest coupling ratios in this group of neurons. Nonetheless, by manipulating their activity, we found that B51 preferentially acted as a driver of B64/B71 activity, whereas B71 played a larger role in driving B8 activity. In contrast, B70 was weakly coupled to other neurons and its inhibition of B8 counteracted the excitatory drive to B8. Finally, the distinct firing patterns of the electrically coupled neurons were fine-tuned by their intrinsic properties and the largely chemical cross-inhibition between some of them. Thus, the small microcircuit of the Aplysia feeding network is advantageous in understanding how a population of electrically coupled heterogeneous neurons may fulfill specific network functions.
Figures





Similar articles
-
B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica.J Neurophysiol. 1996 Apr;75(4):1327-44. doi: 10.1152/jn.1996.75.4.1327. J Neurophysiol. 1996. PMID: 8727381
-
Neural mechanisms of motor program switching in Aplysia.J Neurosci. 2001 Sep 15;21(18):7349-62. doi: 10.1523/JNEUROSCI.21-18-07349.2001. J Neurosci. 2001. PMID: 11549745 Free PMC article.
-
Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica.J Neurophysiol. 1997 Sep;78(3):1305-19. doi: 10.1152/jn.1997.78.3.1305. J Neurophysiol. 1997. PMID: 9310422
-
Feeding neural networks in the mollusc Aplysia.Neurosignals. 2004 Jan-Apr;13(1-2):70-86. doi: 10.1159/000076159. Neurosignals. 2004. PMID: 15004426 Review.
-
Comparative neurobiology of feeding in the opisthobranch sea slug, Aplysia, and the pulmonate snail, Helisoma: evolutionary considerations.Brain Behav Evol. 2009;74(3):219-30. doi: 10.1159/000258668. Epub 2009 Dec 21. Brain Behav Evol. 2009. PMID: 20029185 Free PMC article. Review.
Cited by
-
Discovery of leucokinin-like neuropeptides that modulate a specific parameter of feeding motor programs in the molluscan model, Aplysia.J Biol Chem. 2017 Nov 17;292(46):18775-18789. doi: 10.1074/jbc.M117.795450. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924050 Free PMC article.
-
Identification of three elevenin receptors and roles of elevenin disulfide bond and residues in receptor activation in Aplysia californica.Sci Rep. 2023 May 11;13(1):7662. doi: 10.1038/s41598-023-34596-9. Sci Rep. 2023. PMID: 37169790 Free PMC article.
-
Identification of an allatostatin C signaling system in mollusc Aplysia.Sci Rep. 2022 Jan 24;12(1):1213. doi: 10.1038/s41598-022-05071-8. Sci Rep. 2022. PMID: 35075137 Free PMC article.
-
Electrical coupling and innexin expression in the stomatogastric ganglion of the crab Cancer borealis.J Neurophysiol. 2014 Dec 1;112(11):2946-58. doi: 10.1152/jn.00536.2014. Epub 2014 Sep 10. J Neurophysiol. 2014. PMID: 25210156 Free PMC article.
-
Self-Attention-Based Contextual Modulation Improves Neural System Identification.ArXiv [Preprint]. 2025 Feb 28:arXiv:2406.07843v3. ArXiv. 2025. PMID: 40061115 Free PMC article. Preprint.
References
-
- Baxter DA, Byrne JH. Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning. Learn Mem. 2006;13:669–680. - PubMed
-
- Bennett MV. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. - PubMed
-
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. - PubMed
-
- Bizzi E, Tresch MC, Saltiel P, d'Avella A. New perspectives on spinal motor systems. Nat Rev Neurosci. 2000;1:101–108. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
