Neuroestrogens rapidly regulate sexual motivation but not performance
- PMID: 23283331
- PMCID: PMC3710137
- DOI: 10.1523/JNEUROSCI.2557-12.2013
Neuroestrogens rapidly regulate sexual motivation but not performance
Erratum in
- J Neurosci. 2013 Mar 6;33(10):4623
Abstract
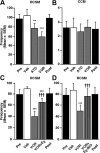
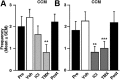
Estrogens exert pleiotropic effects on reproductive traits, which include differentiation and activation of reproductive behaviors and the control of the secretion of gonadotropins. Estrogens also profoundly affect non-reproductive traits, such as cognition and neuroprotection. These effects are usually attributed to nuclear receptor binding and subsequent regulation of target gene transcription. Estrogens also affect neuronal activity and cell-signaling pathways via faster, membrane-initiated events. How these two types of actions that operate in distinct timescales interact in the control of complex behavioral responses is poorly understood. Here, we show that the central administration of estradiol rapidly increases the expression of sexual motivation, as assessed by several measures of sexual motivation produced in response to the visual presentation of a female but not sexual performance in male Japanese quail. This effect is mimicked by membrane-impermeable analogs of estradiol, indicating that it is initiated at the cell membrane. Conversely, blocking the action of estrogens or their synthesis by a single intracerebroventricular injection of estrogen receptor antagonists or aromatase inhibitors, respectively, decreases sexual motivation within minutes without affecting performance. The same steroid has thus evolved complementary mechanisms to regulate different behavioral components (motivation vs performance) in distinct temporal domains (long- vs short-term) so that diverse reproductive activities can be properly coordinated to improve reproductive fitness. Given the pleiotropic effects exerted by estrogens, other responses controlled by these steroids might also depend on a slow genomic regulation of neuronal plasticity underlying behavioral activation and an acute control of motivation to engage in behavior.
Figures

References
-
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. - PubMed
-
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. - PubMed
-
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. - PubMed
-
- Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990a;47:83–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
