Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats
- PMID: 23283335
- PMCID: PMC3711609
- DOI: 10.1523/JNEUROSCI.2016-12.2013
Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats
Abstract
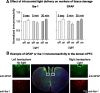
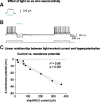
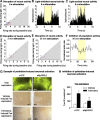
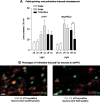
Relapse to maladaptive eating habits during dieting is often provoked by stress. Recently, we identified a role of dorsal medial prefrontal cortex (mPFC) neurons in stress-induced reinstatement of palatable food seeking in male rats. It is unknown whether endogenous neural activity in dorsal mPFC drives stress-induced reinstatement in female rats. Here, we used an optogenetic approach, in which female rats received bilateral dorsal mPFC microinjections of viral constructs coding light-sensitive eNpHR3.0-eYFP or control eYFP protein and intracranial fiber optic implants. Rats were food restricted and trained to lever press for palatable food pellets. Subsequently, pellets were removed, and lever pressing was extinguished; then the effect of bilateral dorsal mPFC light delivery on reinstatement of food seeking was assessed after injections of the pharmacological stressor yohimbine (an α-2 andrenoceptor antagonist) or pellet priming, a manipulation known to provoke food seeking in hungry rats. Dorsal mPFC light delivery attenuated yohimbine-induced reinstatement of food seeking in eNpHR3.0-injected but not eYFP-injected rats. This optical manipulation had no effect on pellet-priming-induced reinstatement or ongoing food-reinforced responding. Dorsal mPFC light delivery attenuated yohimbine-induced Fos immunoreactivity and disrupted neural activity during in vivo electrophysiological recording in awake rats. Optical stimulation caused significant outward currents and blocked electrically evoked action potentials in eNpHR3.0-injected but not eYFP-injected mPFC hemispheres. Light delivery alone caused no significant inflammatory response in mPFC. These findings indicate that intracranial light delivery in eNpHR3.0 rats disrupts endogenous dorsal mPFC neural activity that plays a role in stress-induced relapse to food seeking in female rats.
Figures


References
-
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. - PubMed
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
