A practical and efficient cellular substrate for the generation of induced pluripotent stem cells from adults: blood-derived endothelial progenitor cells
- PMID: 23283547
- PMCID: PMC3659672
- DOI: 10.5966/sctm.2012-0093
A practical and efficient cellular substrate for the generation of induced pluripotent stem cells from adults: blood-derived endothelial progenitor cells
Abstract
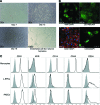
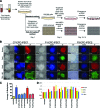
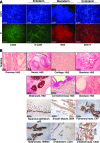
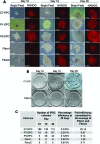
Induced pluripotent stem cells (iPSCs) have the potential to generate patient-specific tissues for disease modeling and regenerative medicine applications. However, before iPSC technology can progress to the translational phase, several obstacles must be overcome. These include uncertainty regarding the ideal somatic cell type for reprogramming, the low kinetics and efficiency of reprogramming, and karyotype discrepancies between iPSCs and their somatic precursors. Here we describe the use of late-outgrowth endothelial progenitor cells (L-EPCs), which possess several favorable characteristics, as a cellular substrate for the generation of iPSCs. We have developed a protocol that allows the reliable isolation of L-EPCs from peripheral blood mononuclear cell preparations, including frozen samples. As a proof-of-principle for clinical applications we generated EPC-iPSCs from both healthy individuals and patients with heritable and idiopathic forms of pulmonary arterial hypertension. L-EPCs grew clonally; were highly proliferative, passageable, and bankable; and displayed higher reprogramming kinetics and efficiencies compared with dermal fibroblasts. Unlike fibroblasts, the high efficiency of L-EPC reprogramming allowed for the reliable generation of iPSCs in a 96-well format, which is compatible with high-throughput platforms. Array comparative genome hybridization analysis of L-EPCs versus donor-matched circulating monocytes demonstrated that L-EPCs have normal karyotypes compared with their subject's reference genome. In addition, >80% of EPC-iPSC lines tested did not acquire any copy number variations during reprogramming compared with their parent L-EPC line. This work identifies L-EPCs as a practical and efficient cellular substrate for iPSC generation, with the potential to address many of the factors currently limiting the translation of this technology.
Figures

References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. - PubMed
-
- Martins-Taylor K, Nisler BS, Taapken SM, et al. Recurrent copy number variations in human induced pluripotent stem cells. Nat Biotechnol. 2011;29:488–491. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

