Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography
- PMID: 23283687
- PMCID: PMC3977617
- DOI: 10.1093/cercor/bhs397
Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography
Abstract
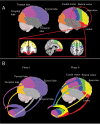
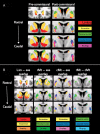
The striatum acts in conjunction with the cortex to control and execute functions that are impaired by abnormal dopamine neurotransmission in disorders such as Parkinson's and schizophrenia. To date, in vivo quantification of striatal dopamine has been restricted to structure-based striatal subdivisions. Here, we present a multimodal imaging approach that quantifies the endogenous dopamine release following the administration of d-amphetamine in the functional subdivisions of the striatum of healthy humans with [(11)C]PHNO and [(11)C]Raclopride positron emission tomography ligands. Using connectivity-based (CB) parcellation, we subdivided the striatum into functional subregions based on striato-cortical anatomical connectivity information derived from diffusion magnetic resonance imaging (MRI) and probabilistic tractography. Our parcellation showed that the functional organization of the striatum was spatially coherent across individuals, congruent with primate data and previous diffusion MRI studies, with distinctive and overlapping networks. d-amphetamine induced the highest dopamine release in the limbic followed by the sensory, motor, and executive areas. The data suggest that the relative regional proportions of D2-like receptors are unlikely to be responsible for this regional dopamine release pattern. Notably, the homogeneity of dopamine release was significantly higher within the CB functional subdivisions in comparison with the structural subdivisions. These results support an association between local levels of dopamine release and cortical connectivity fingerprints.
Keywords: diffusion-weighted image; dopamine receptors; positron emission tomography; probabilistic tractography; striatum.
Figures


References
-
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi:10.1016/S1053-8119(03)00336-7. - DOI - PubMed
-
- Aston JA, Cunningham VJ, Asselin MC, Hammers A, Evans AC, Gunn RN. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002;22:1019–1034. doi:10.1097/00004647-200208000-00014. - DOI - PubMed
-
- Banerjee A, Prante O. Subtype-selective dopamine receptor radioligands for PET imaging: current status and recent developments. Curr Med Chem. 2012;19:3957–3966. doi:10.2174/092986712802002518. - DOI - PubMed
-
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi:10.1038/nn1075. - DOI - PubMed
-
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi:10.1016/j.neuroimage.2006.09.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

