An endothelial cell-specific requirement for the UL133-UL138 locus of human cytomegalovirus for efficient virus maturation
- PMID: 23283945
- PMCID: PMC3592143
- DOI: 10.1128/JVI.02510-12
An endothelial cell-specific requirement for the UL133-UL138 locus of human cytomegalovirus for efficient virus maturation
Abstract
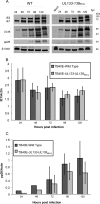
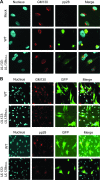
Human cytomegalovirus (HCMV) infects a variety of cell types in humans, resulting in a varied pathogenesis in the immunocompromised host. Endothelial cells (ECs) are considered an important target of HCMV infection that may contribute to viral pathogenesis. Although the viral determinants important for entry into ECs are well defined, the molecular determinants regulating postentry tropism in ECs are not known. We previously identified the UL133-UL138 locus encoded within the clinical strain-specific ULb' region of the HCMV genome as important for the latent infection in CD34(+) hematopoietic progenitor cells (HPCs). Interestingly, this locus, while dispensable for replication in fibroblasts, was required for efficient replication in ECs infected with the TB40E or fusion-inducing factor X (FIX) HCMV strains. ECs infected with a virus lacking the entire locus (UL133-UL138(NULL) virus) complete the immediate-early and early phases of infection but are defective for infectious progeny virus production. ECs infected with UL133-UL138(NULL) virus exhibited striking differences in the organization of intracellular membranes and in the assembly of mature virions relative to ECs infected with wild-type (WT) virus. In UL133-UL138(NULL) virus-infected ECs, Golgi stacks were disrupted, and the viral assembly compartment characteristic of HCMV infection failed to form. Further, progeny virions in UL133-UL138(NULL) virus-infected ECs inefficiently acquired the virion tegument and secondary envelope. These defects were specific to infection in ECs and not observed in fibroblasts infected with UL133-UL138(NULL) virus, suggesting an EC-specific requirement for the UL133-UL138 locus for late stages of replication. To our knowledge, the UL133-UL138 locus represents the first cell-type-dependent, postentry tropism determinant required for viral maturation.
Figures







Similar articles
-
Human Cytomegalovirus UL135 and UL136 Genes Are Required for Postentry Tropism in Endothelial Cells.J Virol. 2015 Jul;89(13):6536-50. doi: 10.1128/JVI.00284-15. Epub 2015 Apr 15. J Virol. 2015. PMID: 25878111 Free PMC article.
-
A novel human cytomegalovirus locus modulates cell type-specific outcomes of infection.PLoS Pathog. 2011 Dec;7(12):e1002444. doi: 10.1371/journal.ppat.1002444. Epub 2011 Dec 29. PLoS Pathog. 2011. PMID: 22241980 Free PMC article.
-
An epistatic relationship between the viral protein kinase UL97 and the UL133-UL138 latency locus during the human cytomegalovirus lytic cycle.J Virol. 2014 Jun;88(11):6047-60. doi: 10.1128/JVI.00447-14. Epub 2014 Mar 12. J Virol. 2014. PMID: 24623439 Free PMC article.
-
The Role of the Human Cytomegalovirus UL133-UL138 Gene Locus in Latency and Reactivation.Viruses. 2020 Jul 1;12(7):714. doi: 10.3390/v12070714. Viruses. 2020. PMID: 32630219 Free PMC article. Review.
-
The power of human cytomegalovirus (HCMV) hijacked UL/b' functions lost in vitro.Acta Virol. 2020;64(2):117-130. doi: 10.4149/av_2020_202. Acta Virol. 2020. PMID: 32551781 Review.
Cited by
-
Human Cytomegalovirus Host Interactions: EGFR and Host Cell Signaling Is a Point of Convergence Between Viral Infection and Functional Changes in Infected Cells.Front Microbiol. 2021 May 7;12:660901. doi: 10.3389/fmicb.2021.660901. eCollection 2021. Front Microbiol. 2021. PMID: 34025614 Free PMC article. Review.
-
Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review.
-
Complex Interplay of the UL136 Isoforms Balances Cytomegalovirus Replication and Latency.mBio. 2016 Mar 1;7(2):e01986. doi: 10.1128/mBio.01986-15. mBio. 2016. PMID: 26933055 Free PMC article.
-
Viral Infection and Ischemic Stroke: Emerging Trends and Mechanistic Insights.J Am Heart Assoc. 2024 Sep 17;13(18):e035892. doi: 10.1161/JAHA.124.035892. Epub 2024 Sep 11. J Am Heart Assoc. 2024. PMID: 39258541 Free PMC article. Review.
-
Human Cytomegalovirus Latency: Approaching the Gordian Knot.Annu Rev Virol. 2016 Sep 29;3(1):333-357. doi: 10.1146/annurev-virology-110615-042422. Epub 2016 Aug 4. Annu Rev Virol. 2016. PMID: 27501258 Free PMC article.
References
-
- Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417–470 - PubMed
-
- Jarvis MA, Nelson JA. 2007. HCMV: molecular basis of persistence and latency, p 780–794 In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom - PubMed
-
- Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2701–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

