Insights into head-tailed viruses infecting extremely halophilic archaea
- PMID: 23283946
- PMCID: PMC3592136
- DOI: 10.1128/JVI.03397-12
Insights into head-tailed viruses infecting extremely halophilic archaea
Abstract
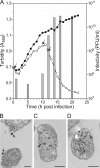
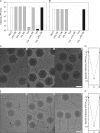
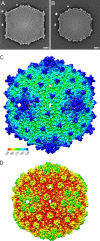
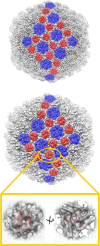
Extremophilic archaea, both hyperthermophiles and halophiles, dominate in habitats where rather harsh conditions are encountered. Like all other organisms, archaeal cells are susceptible to viral infections, and to date, about 100 archaeal viruses have been described. Among them, there are extraordinary virion morphologies as well as the common head-tailed viruses. Although approximately half of the isolated archaeal viruses belong to the latter group, no three-dimensional virion structures of these head-tailed viruses are available. Thus, rigorous comparisons with bacteriophages are not yet warranted. In the present study, we determined the genome sequences of two of such viruses of halophiles and solved their capsid structures by cryo-electron microscopy and three-dimensional image reconstruction. We show that these viruses are inactivated, yet remain intact, at low salinity and that their infectivity is regained when high salinity is restored. This enabled us to determine their three-dimensional capsid structures at low salinity to a ∼10-Å resolution. The genetic and structural data showed that both viruses belong to the same T-number class, but one of them has enlarged its capsid to accommodate a larger genome than typically associated with a T=7 capsid by inserting an additional protein into the capsid lattice.
Figures




References
-
- Chaban B, Ng SYM, Jarrell KF. 2006. Archaeal habitats—from the extreme to the ordinary. Can. J. Microbiol. 52:73–116 - PubMed
-
- DeLong EF, Pace NR. 2001. Environmental diversity of bacteria and archaea. Syst. Biol. 50:470–478 - PubMed
-
- Pina M, Bize A, Forterre P, Prangishvili D. 2011. The archeoviruses. FEMS Microbiol. Rev. 35:1035–1054 - PubMed
-
- Ackermann HW, Prangishvili D. 2012. Prokaryote viruses studied by electron microscopy. Arch. Virol. 157:1843–1849 - PubMed
-
- Atanasova NS, Roine E, Oren A, Bamford DH, Oksanen HM. 2012. Global network of specific virus-host interactions in hypersaline environments. Environ. Microbiol. 14:426–440 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

