Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis
- PMID: 23283952
- PMCID: PMC3592176
- DOI: 10.1128/JVI.02826-12
Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis
Abstract
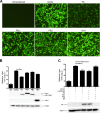
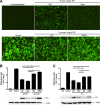
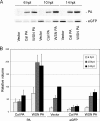
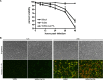
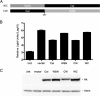
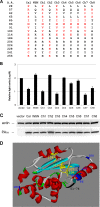
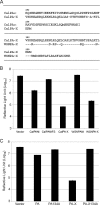
Cellular protein synthesis is suppressed during influenza virus infection, allowing for preferential production of viral proteins. To explore the impact of polymerase subunits on protein synthesis, we coexpressed enhanced green fluorescent protein (eGFP) or luciferase together with each polymerase component or NS1 of A/California/04/2009 (Cal) and found that PA has a significant impact on the expression of eGFP and luciferase. Comparison of the suppressive activity on coexpressed proteins between various strains revealed that avian virus or avian-origin PAs have much stronger activity than human-origin PAs, such as the one from A/WSN/33 (WSN). Protein synthesis data suggested that reduced expression of coexpressed proteins is not due to PA's reported proteolytic activity. A recombinant WSN containing Cal PA showed enhanced host protein synthesis shutoff and induction of apoptosis. Further characterization of the PA fragment indicated that the N-terminal domain (PANt), which includes the endonuclease active site, is sufficient to suppress cotransfected gene expression. By characterizing various chimeric PANts, we found that multiple regions of PA, mainly the helix α4 and the flexible loop of amino acids 51 to 74, affect the activity. The suppressive effect of PANt cDNA was mainly due to PA-X, which was expressed by ribosomal frameshifting. In both Cal and WSN viruses, PA-X showed a stronger effect than the corresponding PANt, suggesting that the unique C-terminal sequences of PA-X also play a role in suppressing cotransfected gene expression. Our data indicate strain variations in PA gene products, which play a major role in suppression of host protein synthesis.
Figures


References
-
- Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. 2006. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 55:1–42 - PubMed
-
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. 2008. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 4:e1000115 doi:10.1371/journal.ppat.1000115 - DOI - PMC - PubMed
-
- Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

