αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion
- PMID: 23283986
- PMCID: PMC3571870
- DOI: 10.1091/mbc.E12-07-0521
αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion
Abstract
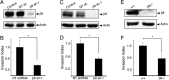
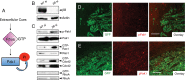
The malignant brain cancer glioblastoma multiforme (GBM) displays invasive growth behaviors that are regulated by extracellular cues within the neural microenvironment. The adhesion and signaling pathways that drive GBM cell invasion remain largely uncharacterized. Here we use human GBM cell lines, primary patient samples, and preclinical mouse models to demonstrate that integrin αvβ8 is a major driver of GBM cell invasion. β8 integrin is overexpressed in many human GBM cells, with higher integrin expression correlating with increased invasion and diminished patient survival. Silencing β8 integrin in human GBM cells leads to impaired tumor cell invasion due to hyperactivation of the Rho GTPases Rac1 and Cdc42. β8 integrin coimmunoprecipitates with Rho-GDP dissociation inhibitor 1 (RhoGDI1), an intracellular signaling effector that sequesters Rho GTPases in their inactive GDP-bound states. Silencing RhoGDI1 expression or uncoupling αvβ8 integrin-RhoGDI1 protein interactions blocks GBM cell invasion due to Rho GTPase hyperactivation. These data reveal for the first time that αvβ8 integrin, via interactions with RhoGDI1, regulates activation of Rho proteins to promote GBM cell invasiveness. Hence targeting the αvβ8 integrin-RhoGDI1 signaling axis might be an effective strategy for blocking GBM cell invasion.
Figures

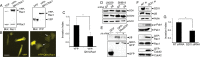
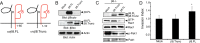

Similar articles
-
The Cytoskeletal Adapter Protein Spinophilin Regulates Invadopodia Dynamics and Tumor Cell Invasion in Glioblastoma.Mol Cancer Res. 2016 Dec;14(12):1277-1287. doi: 10.1158/1541-7786.MCR-16-0251. Epub 2016 Sep 21. Mol Cancer Res. 2016. PMID: 27655131 Free PMC article.
-
MLL regulates the actin cytoskeleton and cell migration by stabilising Rho GTPases via the expression of RhoGDI1.J Cell Sci. 2022 Oct 15;135(20):jcs260042. doi: 10.1242/jcs.260042. Epub 2022 Oct 20. J Cell Sci. 2022. PMID: 36111497 Free PMC article.
-
Cholecystokinin-mediated RhoGDI phosphorylation via PKCα promotes both RhoA and Rac1 signaling.PLoS One. 2013 Jun 11;8(6):e66029. doi: 10.1371/journal.pone.0066029. Print 2013. PLoS One. 2013. PMID: 23776598 Free PMC article.
-
The RHO Family GTPases: Mechanisms of Regulation and Signaling.Cells. 2021 Jul 20;10(7):1831. doi: 10.3390/cells10071831. Cells. 2021. PMID: 34359999 Free PMC article. Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
MiR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1.Oncotarget. 2015 Nov 3;6(34):36231-44. doi: 10.18632/oncotarget.4740. Oncotarget. 2015. PMID: 26460549 Free PMC article.
-
Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: an update.Oncotarget. 2017 Aug 21;8(49):86947-86968. doi: 10.18632/oncotarget.20372. eCollection 2017 Oct 17. Oncotarget. 2017. PMID: 29156849 Free PMC article. Review.
-
Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10161-E10168. doi: 10.1073/pnas.1710680114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109269 Free PMC article.
-
Massive digital gene expression analysis reveals different predictive profiles for immune checkpoint inhibitor therapy between adenocarcinoma and squamous cell carcinoma of advanced lung cancer.BMC Cancer. 2022 Feb 8;22(1):154. doi: 10.1186/s12885-022-09264-2. BMC Cancer. 2022. PMID: 35135489 Free PMC article.
-
Tracking a TGF-β activator in vivo: sensitive PET imaging of αvβ8-integrin with the Ga-68-labeled cyclic RGD octapeptide trimer Ga-68-Triveoctin.EJNMMI Res. 2020 Oct 31;10(1):133. doi: 10.1186/s13550-020-00706-1. EJNMMI Res. 2020. PMID: 33128636 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

