Angiotensin II contributes to glomerular hyperfiltration in diabetic rats independently of adenosine type I receptors
- PMID: 23283998
- PMCID: PMC3602706
- DOI: 10.1152/ajprenal.00285.2012
Angiotensin II contributes to glomerular hyperfiltration in diabetic rats independently of adenosine type I receptors
Abstract
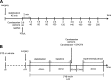
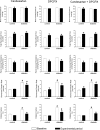
Increased angiotensin II (ANG II) or adenosine can potentiate each other in the regulation of renal hemodynamics and tubular function. Diabetes is characterized by hyperfiltration, yet the roles of ANG II and adenosine receptors for controlling baseline renal blood flow (RBF) or tubular Na(+) handling in diabetes is presently unknown. Accordingly, the changes in their functions were investigated in control and 2-wk streptozotocin-diabetic rats after intrarenal infusion of the ANG II AT1 receptor antagonist candesartan, the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), or their combination. Compared with controls, the baseline blood pressure, RBF, and renal vascular resistance (RVR) were similar in diabetics, whereas the glomerular filtration rate (GFR) and filtration fraction (FF) were increased. Candesartan, DPCPX, or the combination increased RBF and decreased RVR similarly in all groups. In controls, the GFR was increased by DPCPX, but in diabetics, it was decreased by candesartan. The FF was decreased by candesartan and DPCPX, independently. DPCPX caused the most pronounced increase in fractional Na(+) excretion in both controls and diabetics, whereas candesartan or the combination only affected fractional Li(+) excretion in diabetics. These results suggest that RBF, via a unifying mechanism, and tubular function are under strict tonic control of both ANG II and adenosine in both control and diabetic kidneys. Furthermore, increased vascular AT1 receptor activity is a contribution to diabetes-induced hyperfiltration independent of any effect of adenosine A1 receptors.
Figures


References
-
- Aki Y, Tomohiro A, Nishiyama A, Kiyomoto K, Kimura S, Abe Y. Effects of KW-3902, a selective and potent adenosine A1 receptor antagonist, on renal hemodynamics and urine formation in anesthetized dogs. Pharmacology 55: 193–201, 1997 - PubMed
-
- Albino-Teixeira A, Matias A, Polonia J, Azevedo I. Blockade of adenosine receptors causes hypertension and cardiovascular structural changes in the rat. J Hypertens Suppl 9: S196–197, 1991 - PubMed
-
- American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 26, Suppl 1: S33–50, 2003 - PubMed
-
- Awad AS, Webb RL, Carey RM, Siragy HM. Renal nitric oxide production is decreased in diabetic rats and improved by AT1 receptor blockade. J Hypertens 22: 1571–1577, 2004 - PubMed
-
- Barrett RJ, Droppleman DA. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. Am J Physiol Renal Fluid Electrolyte Physiol 265: F651–F659, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

