Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010
- PMID: 23284029
- PMCID: PMC3592060
- DOI: 10.1128/JCM.00923-12
Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010
Abstract
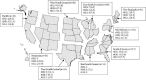
While much is known about the geographic distribution of different clonal types of methicillin-resistant Staphylococcus aureus (MRSA), few studies have assessed the molecular epidemiology of methicillin-susceptible S. aureus (MSSA), despite its continued clinical importance. In each U.S. Census region, reference laboratories collected successive MSSA isolates from patients with invasive or superficial staphylococcal infections for use in the Tigecycline Evaluation and Surveillance Trial. All isolates from the periods of 2004 to 2005 and 2009 to 2010 underwent antimicrobial susceptibility testing and characterization of their staphylococcal protein A (spa) type. Of the 708 isolates analyzed, 274 spa types were identified and divided into 15 genetic clusters. The most common clones were spa t002 (n = 63, 8.9%) and t008 (n = 56, 7.9%). While the distribution of the predominant spa types did not differ by U.S. Census region or time period, spa t008 was nearly twice as common in community skin and soft tissue infections than in nosocomial bloodstream infections (11.1% versus 5.6%, respectively; P = 0.008). Despite such differences, both community and nosocomial settings had diverse staphylococcal clonal types representing all major spa clusters. In contrast to those of MRSA, MSSA infectious isolates show wide genetic diversity without clear geographical or temporal clustering. Notably, the prevalent MSSA strains (spa t002 and spa t008) are analogous to the predominant MRSA clones, further demonstrating the importance of both lineages.
Figures


References
-
- Bouchillon SK, Hoban DJ, Johnson BM, Johnson JL, Hsiung A, Dowzicky MJ, Tigecycline Evaluation and Surveillance Trial (TEST Group) 2005. In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (TEST Program; 2004). Diagn. Microbiol. Infect. Dis. 52:173–179 - PubMed
-
- Cespedes C, Said-Salim B, Miller M, Lo SH, Kreiswirth BN, Gordon RJ, Vavagiakis P, Klein RS, Lowy FD. 2005. The clonality of Staphylococcus aureus nasal carriage. J. Infect. Dis. 191:444–452 - PubMed
-
- Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplemental. M100-S22. CLSI, Wayne, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

