Development of the endochondral skeleton
- PMID: 23284041
- PMCID: PMC3579395
- DOI: 10.1101/cshperspect.a008334
Development of the endochondral skeleton
Abstract
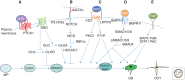
Much of the mammalian skeleton is composed of bones that originate from cartilage templates through endochondral ossification. Elucidating the mechanisms that control endochondral bone development is critical for understanding human skeletal diseases, injury response, and aging. Mouse genetic studies in the past 15 years have provided unprecedented insights about molecules regulating chondrocyte formation, chondrocyte maturation, and osteoblast differentiation, all key processes of endochondral bone development. These include the roles of the secreted proteins IHH, PTHrP, BMPs, WNTs, and FGFs, their receptors, and transcription factors such as SOX9, RUNX2, and OSX, in regulating chondrocyte and osteoblast biology. This review aims to integrate the known functions of extracellular signals and transcription factors that regulate development of the endochondral skeleton.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
