DNA repair by reversal of DNA damage
- PMID: 23284047
- PMCID: PMC3579392
- DOI: 10.1101/cshperspect.a012575
DNA repair by reversal of DNA damage
Erratum in
- Cold Spring Harb Perspect Biol. 2014 Apr;6(4):a023440
Abstract
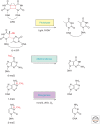
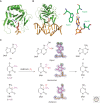
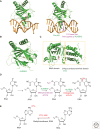
Endogenous and exogenous factors constantly challenge cellular DNA, generating cytotoxic and/or mutagenic DNA adducts. As a result, organisms have evolved different mechanisms to defend against the deleterious effects of DNA damage. Among these diverse repair pathways, direct DNA-repair systems provide cells with simple yet efficient solutions to reverse covalent DNA adducts. In this review, we focus on recent advances in the field of direct DNA repair, namely, photolyase-, alkyltransferase-, and dioxygenase-mediated repair processes. We present specific examples to describe new findings of known enzymes and appealing discoveries of new proteins. At the end of this article, we also briefly discuss the influence of direct DNA repair on other fields of biology and its implication on the discovery of new biology.
Figures

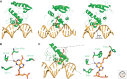
References
-
- Aas PA, Otterlei M, Falnes PO, Vagbo CB, Skorpen F, Akbari M, Sundheim O, Bjoras M, Slupphaug G, Seeberg E, et al. 2003. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421: 859–863 - PubMed
-
- Bleijlevens B, Shivarattan T, van den Boom KS, de Haan A, van der Zwan G, Simpson PJ, Matthews SJ 2012. Changes in protein dynamics of the DNA repair dioxygenase AlkB upon binding of Fe2+ and 2-oxoglutarate. Biochemistry 51: 3334–3341 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
