Metabolic changes in DYT11 myoclonus-dystonia
- PMID: 23284065
- PMCID: PMC3589244
- DOI: 10.1212/WNL.0b013e31827f0798
Metabolic changes in DYT11 myoclonus-dystonia
Abstract
Objective: To identify brain regions with metabolic changes in DYT11 myoclonus-dystonia (DYT11-MD) relative to control subjects and to compare metabolic abnormalities in DYT11-MD with those found in other forms of hereditary dystonia and in posthypoxic myoclonus.
Methods: [(18)F]-fluorodeoxyglucose PET was performed in 6 subjects with DYT11-MD (age 30.5 ± 10.1 years) and in 6 nonmanifesting DYT11 mutation carriers (NM-DYT11; age 59.1 ± 8.9 years) representing the parental generation of the affected individuals. These data were compared to scan data from age-matched healthy control subjects using voxel-based whole brain searches and group differences were considered significant at p < 0.05 (corrected, statistical parametric mapping). As a secondary analysis, overlapping abnormalities were identified by comparisons to hereditary dystonias (DYT1, DYT6, dopa-responsive dystonia) and to posthypoxic myoclonus.
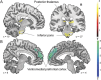
Results: We found significant DYT11 genotype-specific metabolic increases in the inferior pons and in the posterior thalamus as well as reductions in the ventromedial prefrontal cortex. Significant phenotype-related increases were present in the parasagittal cerebellum. This latter abnormality was shared with posthypoxic myoclonus, but not with other forms of dystonia. By contrast, all dystonia cohorts exhibited significant metabolic increases in the superior parietal lobule.
Conclusions: The findings are consistent with a subcortical myoclonus generator in DYT11-MD, likely involving the cerebellum. By contrast, subtle increases in the superior parietal cortex relate to the additional presence of dystonic symptoms. Although reduced penetrance in DYT11-MD has been attributed to the maternal imprinting epsilon-sarcoglycan mutations, NM-DYT11 carriers showed significant metabolic abnormalities that are not explained by this genetic model.
Figures

References
-
- Roze E, Apartis E, Clot F, et al. Myoclonus-dystonia: clinical and electrophysiologic pattern related to SGCE mutations. Neurology 2008;70:1010–1016 - PubMed
-
- Zimprich A, Grabowski M, Asmus F, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet 2001;29:66–69 - PubMed
-
- Valente EM, Edwards MJ, Mir P, et al. The epsilon-sarcoglycan gene in myoclonic syndromes. Neurology 2005;64:737–739 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
