RAC1P29S is a spontaneously activating cancer-associated GTPase
- PMID: 23284172
- PMCID: PMC3549122
- DOI: 10.1073/pnas.1220895110
RAC1P29S is a spontaneously activating cancer-associated GTPase
Abstract
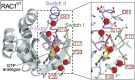
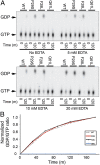
RAC1 is a small, Ras-related GTPase that was recently reported to harbor a recurrent UV-induced signature mutation in melanoma, resulting in substitution of P29 to serine (RAC1(P29S)), ranking this the third most frequently occurring gain-of-function mutation in melanoma. Although the Ras family GTPases are mutated in about 30% of all cancers, mutations in the Rho family GTPases have rarely been observed. In this study, we demonstrate that unlike oncogenic Ras proteins, which are primarily activated by mutations that eliminate GTPase activity, the activated melanoma RAC1(P29S) protein maintains intrinsic GTP hydrolysis and is spontaneously activated by substantially increased inherent GDP/GTP nucleotide exchange. Determination and comparison of crystal structures for activated RAC1 GTPases suggest that RAC1(F28L)--a known spontaneously activated RAC1 mutant--and RAC1(P29S) are self-activated in distinct fashions. Moreover, the mechanism of RAC1(P29S) and RAC1(F28L) activation differs from the common oncogenic mutations found in Ras-like GTPases that abrogate GTP hydrolysis. The melanoma RAC1(P29S) gain-of-function point mutation therefore represents a previously undescribed class of cancer-related GTPase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294(5545):1299–1304. - PubMed
-
- Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
-
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582(14):2093–2101. - PubMed
-
- Ellenbroek SI, Collard JG. Rho GTPases: Functions and association with cancer. Clin Exp Metastasis. 2007;24(8):657–672. - PubMed
-
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(5):682–687. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

