3'-Ferrocenylcarbon-yl-1'-methyl-4'-phenyl-spiro-[indeno-[2,3-b]quinoxaline-11,2'-pyrrolidine]
- PMID: 23284358
- PMCID: PMC3515131
- DOI: 10.1107/S1600536812042468
3'-Ferrocenylcarbon-yl-1'-methyl-4'-phenyl-spiro-[indeno-[2,3-b]quinoxaline-11,2'-pyrrolidine]
Abstract
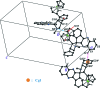
In the title compound, [Fe(C(5)H(5))(C(31)H(24)N(3)O)], the pyrrolidine ring makes a dihedral angle of 86.3 (3)° with the mean plane [r.m.s deviation = 0.074 (2) Å] of the indeno-quinoxaline ring system. The central pyrrolidine ring adopts a twist conformation and the two cyclopentadienyl rings adopt an eclipsed conformation. In the crystal, mol-ecules are linked by weak C-H⋯N and C-H⋯π inter-actions, propagating along the c and a axes, respectively.
Figures

References
-
- Biot, C., Dessolin, J., Richard, I. & Dive, D. (2004). J. Organomet. Chem. 689, 4678–4682.
-
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Fouda, M. F. R., Abd-Elzaher, M. M., Abdelsamaia, R. A. & Labib, A. A. (2007). Appl. Organomet. Chem. 21, 613–625.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
