Activation of TGF-β pathway by areca nut constituents: a possible cause of oral submucous fibrosis
- PMID: 23284772
- PMCID: PMC3526649
- DOI: 10.1371/journal.pone.0051806
Activation of TGF-β pathway by areca nut constituents: a possible cause of oral submucous fibrosis
Abstract
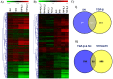
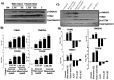
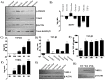
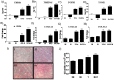
Oral submucous fibrosis (OSF) is a chronic inflammatory disease characterized by the accumulation of excess collagen, and areca nut chewing has been proposed as an important etiological factor for disease manifestation. Activation of transforming growth factor-β signaling has been postulated as the main causative event for increased collagen production in OSF. Oral epithelium plays important roles in OSF, and arecoline has been shown to induce TGF-β in epithelial cells. In an attempt to understand the role of areca nut constituents in the manifestation of OSF, we studied the global gene expression profile in epithelial cells (HaCaT) following treatment with areca nut water extract or TGF-β. Interestingly, 64% of the differentially regulated genes by areca nut water extract matches with the TGF-β induced gene expression profile. Out of these, expression of 57% of genes was compromised in the presence of ALK5 (TβRI) inhibitor and 7% were independently induced by areca nut, highlighting the importance of TGF-β in areca nut actions. Areca nut water extract treatment induced p-SMAD2 and TGF-β downstream targets in HaCaT cells but not in human gingival fibroblast cells (hGF), suggesting epithelial cells could be the source of TGF-β in promoting OSF. Water extract of areca nut consists of polyphenols and alkaloids. Both polyphenol and alkaloid fractions of areca nut were able to induce TGF-β signaling and its downstream targets. Also, SMAD-2 was phosphorylated following treatment of HaCaT cells by Catechin, Tannin and alkaloids namely Arecoline, Arecaidine and Guvacine. Moreover, both polyphenols and alkaloids induced TGF-β2 and THBS1 (activator of latent TGF-β) in HaCaT cells suggesting areca nut mediated activation of p-SMAD2 involves up-regulation and activation of TGF-β. These data suggest a major causative role for TGF-β that is induced by areca nut in OSF progression.
Conflict of interest statement
Figures

Similar articles
-
Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis.PLoS One. 2015 Jun 24;10(6):e0129252. doi: 10.1371/journal.pone.0129252. eCollection 2015. PLoS One. 2015. PMID: 26107172 Free PMC article.
-
Arecoline activates latent transforming growth factor β1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallate.J Formos Med Assoc. 2018 Jun;117(6):527-534. doi: 10.1016/j.jfma.2017.07.003. Epub 2017 Jul 15. J Formos Med Assoc. 2018. PMID: 28720506
-
Oral submucous fibrosis: review on aetiology and pathogenesis.Oral Oncol. 2006 Jul;42(6):561-8. doi: 10.1016/j.oraloncology.2005.08.005. Epub 2005 Nov 28. Oral Oncol. 2006. PMID: 16311067 Review.
-
Panax notoginseng saponins inhibit areca nut extract-induced oral submucous fibrosis in vitro.J Oral Pathol Med. 2014 Jul;43(6):464-70. doi: 10.1111/jop.12158. Epub 2014 Feb 1. J Oral Pathol Med. 2014. PMID: 24484214
-
Areca nut-induced oral fibrosis - Reassessing the biology of oral submucous fibrosis.J Oral Biosci. 2024 Jun;66(2):320-328. doi: 10.1016/j.job.2024.02.005. Epub 2024 Feb 22. J Oral Biosci. 2024. PMID: 38395254 Review.
Cited by
-
TUSC3, p53 and p21 genetic association with development of oral submucous fibrosis and oral squamous cell carcinoma among addictive tobacco chewers of Pakistan.BMC Oral Health. 2024 Jul 11;24(1):780. doi: 10.1186/s12903-024-04501-5. BMC Oral Health. 2024. PMID: 38992585 Free PMC article.
-
The functional roles of microRNAs in the pathogenesis of oral submucous fibrosis.J Dent Sci. 2022 Apr;17(2):683-687. doi: 10.1016/j.jds.2021.07.008. Epub 2021 Aug 1. J Dent Sci. 2022. PMID: 35756801 Free PMC article. Review.
-
Fucoidan-Mediated Inhibition of Fibrotic Properties in Oral Submucous Fibrosis via the MEG3/miR-181a/Egr1 Axis.Pharmaceuticals (Basel). 2022 Jul 5;15(7):833. doi: 10.3390/ph15070833. Pharmaceuticals (Basel). 2022. PMID: 35890132 Free PMC article.
-
Role of programmed cell death 4 in myofibroblast differentiation in oral submucous fibrosis.J Oral Maxillofac Pathol. 2021 Sep-Dec;25(3):430-436. doi: 10.4103/jomfp.jomfp_86_21. Epub 2022 Jan 11. J Oral Maxillofac Pathol. 2021. PMID: 35281179 Free PMC article.
-
Long Non-Coding RNAs in Oral Submucous Fibrosis: Their Functional Mechanisms and Recent Research Progress.J Inflamm Res. 2021 Nov 3;14:5787-5800. doi: 10.2147/JIR.S337014. eCollection 2021. J Inflamm Res. 2021. PMID: 34764671 Free PMC article. Review.
References
-
- Le PV, Gornitsky M, Domanowski G (1996) Oral stent as treatment adjunct for oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 148–150. - PubMed
-
- Sinor PN, Gupta PC, Murti PR, Bhonsle RB, Daftary DK, et al. (1990) A case-control study of oral submucous fibrosis with special reference to the etiologic role of areca nut. J Oral Pathol Med 19: 94–98. - PubMed
-
- Sumeth Perera MW, Gunasinghe D, Perera PA, Ranasinghe A, Amaratunga P, et al. (2007) Development of an in vivo mouse model to study oral submucous fibrosis. J Oral Pathol Med 36: 273–280. - PubMed
-
- Rajalalitha P, Vali S (2005) Molecular pathogenesis of oral submucous fibrosis–a collagen metabolic disorder. J Oral Pathol Med 34: 321–328. - PubMed
-
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, et al. (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

