A mouse model for osseous heteroplasia
- PMID: 23284784
- PMCID: PMC3526487
- DOI: 10.1371/journal.pone.0051835
A mouse model for osseous heteroplasia
Abstract
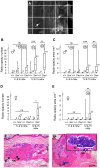
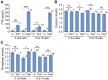
GNAS/Gnas encodes G(s)α that is mainly biallelically expressed but shows imprinted expression in some tissues. In Albright Hereditary Osteodystrophy (AHO) heterozygous loss of function mutations of GNAS can result in ectopic ossification that tends to be superficial and attributable to haploinsufficiency of biallelically expressed G(s)α. Oed-Sml is a point missense mutation in exon 6 of the orthologous mouse locus Gnas. We report here both the late onset ossification and occurrence of benign cutaneous fibroepithelial polyps in Oed-Sml. These phenotypes are seen on both maternal and paternal inheritance of the mutant allele and are therefore due to an effect on biallelically expressed G(s)α. The ossification is confined to subcutaneous tissues and so resembles the ossification observed with AHO. Our mouse model is the first with both subcutaneous ossification and fibroepithelial polyps related to G(s)α deficiency. It is also the first mouse model described with a clinically relevant phenotype associated with a point mutation in G(s)α and may be useful in investigations of the mechanisms of heterotopic bone formation. Together with earlier results, our findings indicate that G(s)α signalling pathways play a vital role in repressing ectopic bone formation.
Conflict of interest statement
Figures


References
-
- Weinstein LS, Yu S, Warner DR, Liu J (2001) Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev 22: 675–705. - PubMed
-
- Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M (2004) Minireview: GNAS: normal and abnormal functions. Endocrinology 145: 5459–5464. - PubMed
-
- Bastepe M, Jüppner H (2005) GNAS locus and pseudohypoparathyroidism. Horm Res 63: 65–74. - PubMed
-
- Yeh GL, Mathur S, Wivel A, Li M, Gannon FH, et al. (2000) GNAS1 mutation and Cbfa1 misexpression in a child with severe congenital platelike osteoma cutis. J Bone Miner Res 15: 2063–2073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

