Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction
- PMID: 23284842
- PMCID: PMC3527411
- DOI: 10.1371/journal.pone.0051991
Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction
Abstract
Background: To improve the efficacy of bone marrow-derived mesenchymal stem cell (MSC) therapy targeted to infarcted myocardium, we investigated whether a self-setting silanized hydroxypropyl methylcellulose (Si-HPMC) hydrogel seeded with MSC (MSC+hydrogel) could preserve cardiac function and attenuate left ventricular (LV) remodeling during an 8-week follow-up study in a rat model of myocardial infarction (MI).
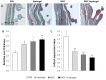
Methodology/principal finding: Si-HPMC hydrogel alone, MSC alone or MSC+hydrogel were injected into the myocardium immediately after coronary artery ligation in female Lewis rats. Animals in the MSC+hydrogel group showed an increase in cardiac function up to 28 days after MI and a mid-term prevention of cardiac function alteration at day 56. Histological analyses indicated that the injection of MSC+hydrogel induced a decrease in MI size and an increase in scar thickness and ultimately limited the transmural extent of MI. These findings show that intramyocardial injection of MSC+hydrogel induced short-term recovery of ventricular function and mid-term attenuation of remodeling after MI.
Conclusion/significance: These beneficial effects may be related to the specific scaffolding properties of the Si-HPMC hydrogel that may provide the ability to support MSC injection and engraftment within myocardium.
Conflict of interest statement
Figures
Similar articles
-
Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium.Circ Res. 2010 Jun 11;106(11):1753-62. doi: 10.1161/CIRCRESAHA.109.196030. Epub 2010 Apr 8. Circ Res. 2010. PMID: 20378860 Free PMC article.
-
Timing effect of intramyocardial hydrogel injection for positively impacting left ventricular remodeling after myocardial infarction.Biomaterials. 2016 Mar;83:182-93. doi: 10.1016/j.biomaterials.2015.12.002. Epub 2015 Dec 15. Biomaterials. 2016. PMID: 26774561 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles alone or in conjunction with a SDKP-conjugated self-assembling peptide improve a rat model of myocardial infarction.Biochem Biophys Res Commun. 2020 Apr 16;524(4):903-909. doi: 10.1016/j.bbrc.2020.02.009. Epub 2020 Feb 10. Biochem Biophys Res Commun. 2020. PMID: 32057366
-
Research and application of hydrogel-encapsulated mesenchymal stem cells in the treatment of myocardial infarction.Colloids Surf B Biointerfaces. 2024 Jul;239:113942. doi: 10.1016/j.colsurfb.2024.113942. Epub 2024 May 6. Colloids Surf B Biointerfaces. 2024. PMID: 38729022 Review.
-
Cardiac cell therapy: boosting mesenchymal stem cells effects.Stem Cell Rev Rep. 2013 Jun;9(3):266-80. doi: 10.1007/s12015-012-9353-z. Stem Cell Rev Rep. 2013. PMID: 22350458 Review.
Cited by
-
Bioactivity of CD34+ cells in patients with acute-on-chronic liver failure.Int J Clin Exp Pathol. 2017 Nov 1;10(11):10781-10791. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966421 Free PMC article.
-
Quantifying Oxygen Levels in 3D Bioprinted Cell-Laden Thick Constructs with Perfusable Microchannel Networks.Polymers (Basel). 2020 May 30;12(6):1260. doi: 10.3390/polym12061260. Polymers (Basel). 2020. PMID: 32486307 Free PMC article.
-
Cardiac mesenchymal cells from failing and nonfailing hearts limit ventricular dilation when administered late after infarction.Am J Physiol Heart Circ Physiol. 2020 Jul 1;319(1):H109-H122. doi: 10.1152/ajpheart.00114.2020. Epub 2020 May 22. Am J Physiol Heart Circ Physiol. 2020. PMID: 32442025 Free PMC article.
-
Experimental and Computational Insight Into Human Mesenchymal Stem Cell Paracrine Signaling and Heterocellular Coupling Effects on Cardiac Contractility and Arrhythmogenicity.Circ Res. 2017 Aug 4;121(4):411-423. doi: 10.1161/CIRCRESAHA.117.310796. Epub 2017 Jun 22. Circ Res. 2017. PMID: 28642329 Free PMC article.
-
The role of adipose-derived stromal cells and hydroxypropylmethylcellulose in engineering cartilage tissue in vivo.Cytotechnology. 2014 Oct;66(5):779-90. doi: 10.1007/s10616-013-9627-6. Epub 2013 Nov 28. Cytotechnology. 2014. PMID: 24287610 Free PMC article.
References
-
- Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35: 569–582. - PubMed
-
- Jugdutt BI (2003) Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation 108: 1395–1403. - PubMed
-
- Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, et al. (2002) Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation 106: 2351–2357. - PubMed
-
- Savoye C, Equine O, Tricot O, Nugue O, Segrestin B, et al. (2006) Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am J Cardiol 98: 1144–1149. - PubMed
-
- Verma A, Meris A, Skali H, Ghali JK, Arnold JM, et al. (2008) Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 1: 582–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

