Rescue of murine F508del CFTR activity in native intestine by low temperature and proteasome inhibitors
- PMID: 23284872
- PMCID: PMC3528711
- DOI: 10.1371/journal.pone.0052070
Rescue of murine F508del CFTR activity in native intestine by low temperature and proteasome inhibitors
Abstract
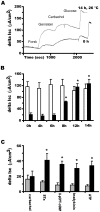
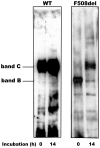
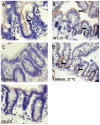
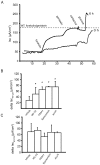
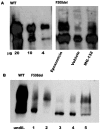
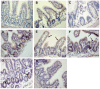
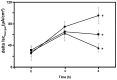
Most patients with Cystic Fibrosis (CF) carry at least one allele with the F508del mutation, resulting in a CFTR chloride channel protein with a processing, gating and stability defect, but with substantial residual activity when correctly sorted to the apical membranes of epithelial cells. New therapies are therefore aimed at improving the folding and trafficking of F508del CFTR, (CFTR correctors) or at enhancing the open probability of the CFTR chloride channel (CFTR potentiators). Preventing premature breakdown of F508del CFTR is an alternative or additional strategy, which is investigated in this study. We established an ex vivo assay for murine F508del CFTR rescue in native intestinal epithelium that can be used as a pre-clinical test for candidate therapeutics. Overnight incubation of muscle stripped ileum in modified William's E medium at low temperature (26°C), and 4 h or 6 h incubation at 37°C with different proteasome inhibitors (PI: ALLN, MG-132, epoxomicin, PS341/bortezomib) resulted in fifty to hundred percent respectively of the wild type CFTR mediated chloride secretion (forskolin induced short-circuit current). The functional rescue was accompanied by enhanced expression of the murine F508del CFTR protein at the apical surface of intestinal crypts and a gain in the amount of complex-glycosylated CFTR (band C) up to 20% of WT levels. Sustained rescue in the presence of brefeldin A shows the involvement of a post-Golgi compartment in murine F508del CFTR degradation, as was shown earlier for its human counterpart. Our data show that proteasome inhibitors are promising candidate compounds for improving rescue of human F508del CFTR function, in combination with available correctors and potentiators.
Conflict of interest statement
Figures
Similar articles
-
Rescue of epithelial HCO3- secretion in murine intestine by apical membrane expression of the cystic fibrosis transmembrane conductance regulator mutant F508del.J Physiol. 2012 Nov 1;590(21):5317-34. doi: 10.1113/jphysiol.2012.232124. Epub 2012 Jul 16. J Physiol. 2012. PMID: 22802588 Free PMC article.
-
Correction of chloride transport and mislocalization of CFTR protein by vardenafil in the gastrointestinal tract of cystic fibrosis mice.PLoS One. 2013 Oct 24;8(10):e77314. doi: 10.1371/journal.pone.0077314. eCollection 2013. PLoS One. 2013. PMID: 24204804 Free PMC article.
-
Unravelling the Regions of Mutant F508del-CFTR More Susceptible to the Action of Four Cystic Fibrosis Correctors.Int J Mol Sci. 2019 Nov 1;20(21):5463. doi: 10.3390/ijms20215463. Int J Mol Sci. 2019. PMID: 31683989 Free PMC article.
-
Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis.Acta Pharmacol Sin. 2011 Jun;32(6):693-701. doi: 10.1038/aps.2011.71. Acta Pharmacol Sin. 2011. PMID: 21642944 Free PMC article. Review.
-
F508del-cystic fibrosis transmembrane regulator correctors for treatment of cystic fibrosis: a patent review.Expert Opin Ther Pat. 2015;25(9):991-1002. doi: 10.1517/13543776.2015.1045878. Epub 2015 May 15. Expert Opin Ther Pat. 2015. PMID: 25971311 Review.
Cited by
-
KCNC3(R420H), a K(+) channel mutation causative in spinocerebellar ataxia 13 displays aberrant intracellular trafficking.Neurobiol Dis. 2014 Nov;71:270-9. doi: 10.1016/j.nbd.2014.08.020. Epub 2014 Aug 22. Neurobiol Dis. 2014. PMID: 25152487 Free PMC article.
-
A connexin50 mutant, CX50fs, that causes cataracts is unstable, but is rescued by a proteasomal inhibitor.J Biol Chem. 2013 Jul 12;288(28):20427-34. doi: 10.1074/jbc.M113.452847. Epub 2013 May 17. J Biol Chem. 2013. PMID: 23720739 Free PMC article.
-
Integrative chemogenomic analysis identifies small molecules that partially rescue ΔF508-CFTR for cystic fibrosis.CPT Pharmacometrics Syst Pharmacol. 2021 May;10(5):500-510. doi: 10.1002/psp4.12626. Epub 2021 May 2. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33934548 Free PMC article.
-
Proteostasis Regulation in the Endoplasmic Reticulum: An Emerging Theme in the Molecular Pathology and Therapeutic Management of Familial Hypercholesterolemia.Front Genet. 2020 Sep 23;11:570355. doi: 10.3389/fgene.2020.570355. eCollection 2020. Front Genet. 2020. PMID: 33173538 Free PMC article. Review.
-
Resveratrol increases F508del-CFTR dependent salivary secretion in cystic fibrosis mice.Biol Open. 2015 Jun 19;4(7):929-36. doi: 10.1242/bio.010967. Biol Open. 2015. PMID: 26092868 Free PMC article.
References
-
- Kartner N, Augustinas O, Jensen TJ, Naismith AL, Riordan JR (1992) Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat Genet 1: 321–327. - PubMed
-
- Trezise AE, Buchwald M (1991) In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 353: 434–437. - PubMed
-
- Bobadilla JL, Macek M Jr, Fine JP, Farrell PM (2002) Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Hum Mutat 19: 575–606. - PubMed
-
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, et al. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

