Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction
- PMID: 23284892
- PMCID: PMC3527406
- DOI: 10.1371/journal.pone.0052120
Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction
Abstract
Aims: Myocardial CCN2/CTGF is induced in heart failure of various etiologies. However, its role in the pathophysiology of left ventricular (LV) remodeling after myocardial infarction (MI) remains unresolved. The current study explores the role of CTGF in infarct healing and LV remodeling in an animal model and in patients admitted for acute ST-elevation MI.
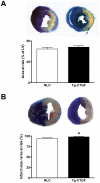
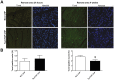
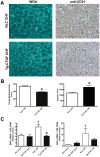
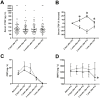
Methods and results: Transgenic mice with cardiac-restricted overexpression of CTGF (Tg-CTGF) and non-transgenic littermate controls (NLC) were subjected to permanent ligation of the left anterior descending coronary artery. Despite similar infarct size (area of infarction relative to area at risk) 24 hours after ligation of the coronary artery in Tg-CTGF and NLC mice, Tg-CTGF mice disclosed smaller area of scar tissue, smaller increase of cardiac hypertrophy, and less LV dilatation and deterioration of LV function 4 weeks after MI. Tg-CTGF mice also revealed substantially reduced mortality after MI. Remote/peri-infarct tissue of Tg-CTGF mice contained reduced numbers of leucocytes, macrophages, and cells undergoing apoptosis as compared with NLC mice. In a cohort of patients with acute ST-elevation MI (n = 42) admitted to hospital for percutaneous coronary intervention (PCI) serum-CTGF levels (s-CTGF) were monitored and related to infarct size and LV function assessed by cardiac MRI. Increase in s-CTGF levels after MI was associated with reduced infarct size and improved LV ejection fraction one year after MI, as well as attenuated levels of CRP and GDF-15.
Conclusion: Increased myocardial CTGF activities after MI are associated with attenuation of LV remodeling and improved LV function mediated by attenuation of inflammatory responses and inhibition of apoptosis.
Conflict of interest statement
Figures





References
-
- Ørn S, Manhenke C, Anand IS, Squire I, Nagel E, et al. (2007) Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol 99: 1109–1114. - PubMed
-
- Ahmed MS, Øie E, Vinge LE, Yndestad A, Andersen GØ, et al. (2004) Connective tissue growth factor - a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol 36: 393–404. - PubMed
-
- Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH (2000) CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32: 1805–1819. - PubMed
-
- Koitabashi N, Arai M, Niwano K, Watanabe A, Endoh M, et al. (2008) Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail 10: 373–379. - PubMed
-
- Shi-Wen X, Leask A, Abraham D (2008) Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine & growth factor reviews 19: 133–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

