High drug resistance prevalence among vertically HIV-infected patients transferred from pediatric care to adult units in Spain
- PMID: 23284913
- PMCID: PMC3524105
- DOI: 10.1371/journal.pone.0052155
High drug resistance prevalence among vertically HIV-infected patients transferred from pediatric care to adult units in Spain
Abstract
Background: Antiretroviral treatment (ART) has contributed to increased life expectancy of HIV-1 infected children. In developed countries, an increasing number of children reaching adulthood are transferred to adult units. The objectives were to describe the demographic and clinical features, ART history, antiviral drug resistance and drug susceptibility in HIV-1 perinatally infected adolescents transferred to adult care units in Spain from the Madrid Cohort of HIV-1 infected children.
Methods: Clinical, virological and immunological features of HIV-1 vertically infected patients in the Madrid Cohort of HIV-infected children were analyzed at the time of transfer. Pol sequences from each patient were recovered before transfer. Resistance mutations according to the InternationaI AIDS Society 2011 list were identified and interpreted using the Stanford algorithm. Results were compared to the non-transferred HIV-1 infected pediatric cohort from Madrid.
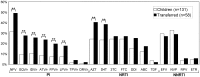
Results: One hundred twelve infected patients were transferred to adult units between 1997 and 2011. They were mainly perinatally infected (93.7%), with a mean nadir CD4+-T-cells count of 10% and presented moderate or severe clinical symptoms (75%). By the time of transfer, the mean age was 18.9 years, the mean CD4+T-cells count was 627.5 cells/ml, 64.2% presented more than 350 CD4+T-cells/ml and 47.3% had ≤ 200 RNA-copies/ml. Most (97.3%) were ART experienced receiving Highly Active ART (HAART) (84.8%). Resistance prevalence among pretreated was 50.9%, 76.9% and 36.5% for Protease Inhibitors (PI), Nucleoside Reverse Transcriptase Inhibitors (NRTI) and Non-NRTI (NNRTI), respectively. Resistance mutations were significantly higher among transferred patients compared to non-transferred for the PI+NRTI combination (19% vs. 8.4%). Triple resistance was similar to non-transferred pediatric patients (17.3% vs. 17.6%).
Conclusion: Despite a good immunological and virological control before transfer, we found high levels of resistance to PI, NRTI and triple drug resistance in HIV-1 infected adolescents transferred to adult units.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO) Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. Available: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf. Accessed 2012 Nov 16.
-
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2010. Stockholm: European Centre for Disease Prevention and Control; 2011. Available: http://ecdc.europa.eu/en/publications/Publications/1111_SUR_Annual_Epide.... Accessed 2012 Nov 16.
-
- European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis 40: 458–465. - PubMed
-
- Welch S, Sharland M, Lyall EG, Tudor-Williams G, Niehues T, et al. (2009) PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med 10: 591–613. - PubMed
-
- US Public Health Service Task Force. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available: http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf. Accessed 2012 Nov 16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

