Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis
- PMID: 23284936
- PMCID: PMC3527399
- DOI: 10.1371/journal.pone.0052208
Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis
Abstract
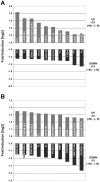
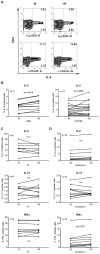
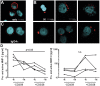
Natalizumab is a recombinant monoclonal antibody raised against integrin alpha-4 (CD49d). It is approved for the treatment of patients with multiple sclerosis (MS), a chronic inflammatory autoimmune disease of the CNS. While having shown high therapeutic efficacy, treatment by natalizumab has been linked to progressive multifocal leukoencephalopathy (PML) as a serious adverse effect. Furthermore, drug cessation sometimes induces rebound disease activity of unknown etiology. Here we investigated whether binding of this adhesion-blocking antibody to T lymphocytes could modulate their phenotype by direct induction of intracellular signaling events. Primary CD4(+) T lymphocytes either from healthy donors and treated with natalizumab in vitro or from MS patients receiving their very first dose of natalizumab were analyzed. Natalizumab induced a mild upregulation of IL-2, IFN-γ and IL-17 expression in activated primary human CD4(+) T cells propagated ex vivo from healthy donors, consistent with a pro-inflammatory costimulatory effect on lymphokine expression. Along with this, natalizumab binding triggered rapid MAPK/ERK phosphorylation. Furthermore, it decreased CD49d surface expression on effector cells within a few hours. Sustained CD49d downregulation could be attributed to integrin internalization and degradation. Importantly, also CD4(+) T cells from some MS patients receiving their very first dose of natalizumab produced more IL-2, IFN-γ and IL-17 already 24 h after infusion. Together these data indicate that in addition to its adhesion-blocking mode of action natalizumab possesses mild direct signaling capacities, which can support a pro-inflammatory phenotype of peripheral blood T lymphocytes. This might explain why a rebound of disease activity or IRIS is observed in some MS patients after natalizumab cessation.
Conflict of interest statement
Figures




References
-
- Compston A, Coles A (2002) Multiple sclerosis. Lancet 359: 1221–1231. - PubMed
-
- Hohlfeld R, Wekerle H (2001) Immunological update on multiple sclerosis. Curr Opin Neurol 14: 299–304. - PubMed
-
- Korn T (2008) Pathophysiology of multiple sclerosis. J Neurol 255 Suppl 62–6. - PubMed
-
- Benvenuto R, Paroli M, Buttinelli C, Franco A, Barnaba V, et al. (1992) Tumor necrosis factor-alpha and interferon-gamma synthesis by cerebrospinal fluid-derived T cell clones in multiple sclerosis. Ann N Y Acad Sci 650: 341–346. - PubMed
-
- Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, et al. (1993) T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity 15: 137–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

