Nippostrongylus-induced intestinal hypercontractility requires IL-4 receptor alpha-responsiveness by T cells in mice
- PMID: 23284939
- PMCID: PMC3527412
- DOI: 10.1371/journal.pone.0052211
Nippostrongylus-induced intestinal hypercontractility requires IL-4 receptor alpha-responsiveness by T cells in mice
Abstract
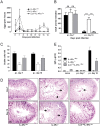
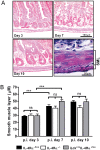
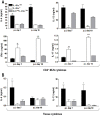
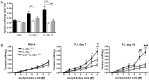
Gut-dwelling helminthes induce potent IL-4 and IL-13 dominated type 2 T helper cell (T(H)2) immune responses, with IL-13 production being essential for Nippostrongylus brasiliensis expulsion. This T(H)2 response results in intestinal inflammation associated with local infiltration by T cells and macrophages. The resulting increased IL-4/IL-13 intestinal milieu drives goblet cell hyperplasia, alternative macrophage activation and smooth muscle cell hypercontraction. In this study we investigated how IL-4-promoted T cells contributed to the parasite induced effects in the intestine. This was achieved using pan T cell-specific IL-4 receptor alpha-deficient mice (iLck(cre)IL-4Rα(-/lox)) and IL-4Rα-responsive control mice. Global IL-4Rα(-/-) mice showed, as expected, impaired type 2 immunity to N. brasiliensis. Infected T cell-specific IL-4Rα-deficient mice showed comparable worm expulsion, goblet cell hyperplasia and IgE responses to control mice. However, impaired IL-4-promoted T(H)2 cells in T cell-specific IL-4Rα deficient mice led to strikingly reduced IL-4 production by mesenteric lymph node CD4(+) T cells and reduced intestinal IL-4 and IL-13 levels, compared to control mice. This reduced IL-4/IL-13 response was associated with an impaired IL-4/IL-13-mediated smooth muscle cell hypercontractility, similar to that seen in global IL-4Rα(-/-) mice. These results demonstrate that IL-4-promoted T cell responses are not required for the resolution of a primary N. brasiliensis infection. However, they do contribute significantly to an important physiological manifestation of helminth infection; namely intestinal smooth muscle cell-driven hypercontractility.
Conflict of interest statement
Figures
Similar articles
-
Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice.PLoS Pathog. 2007 Jan;3(1):e1. doi: 10.1371/journal.ppat.0030001. PLoS Pathog. 2007. PMID: 17222057 Free PMC article. Clinical Trial.
-
IL-4Rα-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections.Mucosal Immunol. 2011 Jan;4(1):83-92. doi: 10.1038/mi.2010.46. Epub 2010 Aug 25. Mucosal Immunol. 2011. PMID: 20737001
-
IL-4R{alpha}-responsive smooth muscle cells increase intestinal hypercontractility and contribute to resistance during acute Schistosomiasis.Am J Physiol Gastrointest Liver Physiol. 2010 Jun;298(6):G943-51. doi: 10.1152/ajpgi.00321.2009. Epub 2010 Apr 1. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20360135
-
Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology.Infect Immun. 2008 Dec;76(12):5535-42. doi: 10.1128/IAI.00210-08. Epub 2008 Sep 22. Infect Immun. 2008. PMID: 18809669 Free PMC article.
-
Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.Immunol Rev. 2004 Oct;201:139-55. doi: 10.1111/j.0105-2896.2004.00192.x. Immunol Rev. 2004. PMID: 15361238 Review.
Cited by
-
the intestinal expulsion of the roundworm Ascaris suum is associated with eosinophils, intra-epithelial T cells and decreased intestinal transit time.PLoS Negl Trop Dis. 2013 Dec 5;7(12):e2588. doi: 10.1371/journal.pntd.0002588. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24340121 Free PMC article.
-
Macrophages in intestinal homeostasis and inflammation.Immunol Rev. 2014 Jul;260(1):102-17. doi: 10.1111/imr.12192. Immunol Rev. 2014. PMID: 24942685 Free PMC article. Review.
-
Impact of Helminth Infections on Female Reproductive Health and Associated Diseases.Front Immunol. 2020 Nov 23;11:577516. doi: 10.3389/fimmu.2020.577516. eCollection 2020. Front Immunol. 2020. PMID: 33329545 Free PMC article. Review.
-
Helminth infection driven gastrointestinal hypermotility is independent of eosinophils and mediated by alterations in smooth muscle instead of enteric neurons.PLoS Pathog. 2024 Aug 14;20(8):e1011766. doi: 10.1371/journal.ppat.1011766. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39141685 Free PMC article.
-
Immunity to gastrointestinal nematode infections.Mucosal Immunol. 2018 Mar;11(2):304-315. doi: 10.1038/mi.2017.113. Epub 2018 Jan 3. Mucosal Immunol. 2018. PMID: 29297502 Review.
References
-
- Brombacher F (2000) The role of interleukin-13 in infectious diseases and allergy. Bioessays 22: 646–656. - PubMed
-
- Zurawski G, de Vries JE (1994) Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today 15: 19–26. - PubMed
-
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701–738. - PubMed
-
- Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, et al. (1986) B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol 136: 4538–4541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

