In vivo human left-to-right ventricular differences in rate adaptation transiently increase pro-arrhythmic risk following rate acceleration
- PMID: 23284948
- PMCID: PMC3527395
- DOI: 10.1371/journal.pone.0052234
In vivo human left-to-right ventricular differences in rate adaptation transiently increase pro-arrhythmic risk following rate acceleration
Abstract
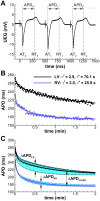
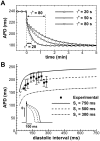
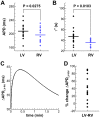
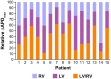
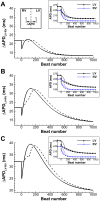
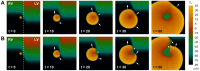
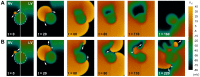
Left-to-right ventricular (LV/RV) differences in repolarization have been implicated in lethal arrhythmias in animal models. Our goal is to quantify LV/RV differences in action potential duration (APD) and APD rate adaptation and their contribution to arrhythmogenic substrates in the in vivo human heart using combined in vivo and in silico studies. Electrograms were acquired from 10 LV and 10 RV endocardial sites in 15 patients with normal ventricles. APD and APD adaptation were measured during an increase in heart rate. Analysis of in vivo electrograms revealed longer APD in LV than RV (207.8 ± 21.5 vs 196.7 ± 20.1 ms; P<0.05), and slower APD adaptation in LV than RV (time constant τ(s) =47.0 ± 14.3 vs 35.6 ± 6.5 s; P<0.05). Following rate acceleration, LV/RV APD dispersion experienced an increase of up to 91% in 12 patients, showing a strong correlation (r(2) =0.90) with both initial dispersion and LV/RV difference in slow adaptation. Pro-arrhythmic implications of measured LV/RV functional differences were studied using in silico simulations. Results show that LV/RV APD and APD adaptation heterogeneities promote unidirectional block following rate acceleration, albeit being insufficient for establishment of reentry in normal hearts. However, in the presence of an ischemic region at the LV/RV junction, LV/RV heterogeneity in APD and APD rate adaptation promotes reentrant activity and its degeneration into fibrillatory activity. Our results suggest that LV/RV heterogeneities in APD adaptation cause a transient increase in APD dispersion in the human ventricles following rate acceleration, which promotes unidirectional block and wave-break at the LV/RV junction, and may potentiate the arrhythmogenic substrate, particularly in patients with ischemic heart disease.
Conflict of interest statement
Figures
References
-
- Kuo CS, Munakata K, Reddy CP, Surawicz B (1983) Characteristics and possible mechanisms of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation 67: 1356–1357. - PubMed
-
- Morgan JM, Cunningham D, Rowland E (1992) Dispersion of monophasic action potential duration: demonstrable in humans after premature ventricular extrastimulation but not in steady state. J Am Coll Cardiol 19: 1244–1253. - PubMed
-
- Yuan S, Wohlfart B, Olsson SB, Blomstrom-Lundqvist C (1995) The dispersion of repolarization in patients with ventricular tachycardia. A study using simultaneous monophasic action potential recordings from two sites in the right ventricle. Eur Heart J 16: 68–76. - PubMed
-
- Nash MP, Bradley CP, Sutton PM, Clayton RH, Kallis P, et al. (2006) Whole heart action potential duration restitution properties in cardiac patients: a combined clinical and modelling study. Exp Physiol 91: 339–354. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

