Vaccination with L. infantum chagasi nucleosomal histones confers protection against new world cutaneous leishmaniasis caused by Leishmania braziliensis
- PMID: 23284976
- PMCID: PMC3527524
- DOI: 10.1371/journal.pone.0052296
Vaccination with L. infantum chagasi nucleosomal histones confers protection against new world cutaneous leishmaniasis caused by Leishmania braziliensis
Abstract
Background: Nucleosomal histones are intracellular proteins that are highly conserved among Leishmania species. After parasite destruction or spontaneous lysis, exposure to these proteins elicits a strong host immune response. In the present study, we analyzed the protective capability of Leishmania infantum chagasi nucleosomal histones against L. braziliensis infection using different immunization strategies.
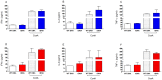
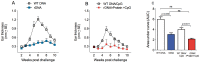
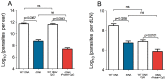
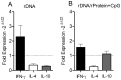
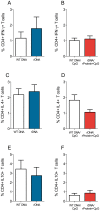
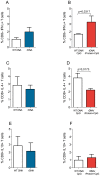
Methodology/principal findings: BALB/c mice were immunized with either a plasmid DNA cocktail (DNA) containing four Leishmania nucleosomal histones or with the DNA cocktail followed by the corresponding recombinant proteins plus CpG (DNA/Protein). Mice were later challenged with L. braziliensis, in the presence of sand fly saliva. Lesion development, parasite load and the cellular immune response were analyzed five weeks after challenge. Immunization with either DNA alone or with DNA/Protein was able to inhibit lesion development. This finding was highlighted by the absence of infected macrophages in tissue sections. Further, parasite load at the infection site and in the draining lymph nodes was also significantly lower in vaccinated animals. This outcome was associated with increased expression of IFN-γ and down regulation of IL-4 at the infection site.
Conclusion: The data presented here demonstrate the potential use of L. infantum chagasi nucleosomal histones as targets for the development of vaccines against infection with L. braziliensis, as shown by the significant inhibition of disease development following a live challenge.
Conflict of interest statement
Figures

Similar articles
-
Vaccination with Leishmania infantum acidic ribosomal P0 but not with nucleosomal histones proteins controls Leishmania infantum infection in hamsters.PLoS Negl Trop Dis. 2015 Feb 2;9(2):e0003490. doi: 10.1371/journal.pntd.0003490. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25642946 Free PMC article.
-
Towards development of novel immunization strategies against leishmaniasis using PLGA nanoparticles loaded with kinetoplastid membrane protein-11.Int J Nanomedicine. 2012;7:2115-27. doi: 10.2147/IJN.S30093. Epub 2012 Apr 24. Int J Nanomedicine. 2012. PMID: 22619548 Free PMC article.
-
Cross-protective effect of a combined L5 plus L3 Leishmania major ribosomal protein based vaccine combined with a Th1 adjuvant in murine cutaneous and visceral leishmaniasis.Parasit Vectors. 2014 Jan 2;7:3. doi: 10.1186/1756-3305-7-3. Parasit Vectors. 2014. PMID: 24382098 Free PMC article.
-
Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis.Vaccine. 2004 Sep 28;22(29-30):3865-76. doi: 10.1016/j.vaccine.2004.04.015. Vaccine. 2004. PMID: 15364433
-
Challenges and perspectives in vaccination against leishmaniasis.Parasitol Int. 2009 Dec;58(4):319-24. doi: 10.1016/j.parint.2009.07.013. Epub 2009 Aug 19. Parasitol Int. 2009. PMID: 19698801 Review.
Cited by
-
Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis.Hum Vaccin Immunother. 2020 Apr 2;16(4):919-930. doi: 10.1080/21645515.2019.1678998. Epub 2019 Nov 11. Hum Vaccin Immunother. 2020. PMID: 31634036 Free PMC article. Review.
-
Gene Expression Profile of High IFN-γ Producers Stimulated with Leishmania braziliensis Identifies Genes Associated with Cutaneous Leishmaniasis.PLoS Negl Trop Dis. 2016 Nov 21;10(11):e0005116. doi: 10.1371/journal.pntd.0005116. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27870860 Free PMC article.
-
In silico and in vitro Evaluation of Mimetic Peptides as Potential Antigen Candidates for Prophylaxis of Leishmaniosis.Front Chem. 2021 Jan 15;8:601409. doi: 10.3389/fchem.2020.601409. eCollection 2020. Front Chem. 2021. PMID: 33520931 Free PMC article.
-
Vaccination with Leishmania infantum acidic ribosomal P0 but not with nucleosomal histones proteins controls Leishmania infantum infection in hamsters.PLoS Negl Trop Dis. 2015 Feb 2;9(2):e0003490. doi: 10.1371/journal.pntd.0003490. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25642946 Free PMC article.
-
Immunoinformatics Approach to Design a Multi-Epitope Nanovaccine against Leishmania Parasite: Elicitation of Cellular Immune Responses.Vaccines (Basel). 2023 Jan 30;11(2):304. doi: 10.3390/vaccines11020304. Vaccines (Basel). 2023. PMID: 36851182 Free PMC article.
References
-
- de Oliveira CI, Nascimento IP, Barral A, Soto M, Barral-Netto M (2009) Challenges and perspectives in vaccination against leishmaniasis. Parasitol Int 58: 319–324. - PubMed
-
- Okwor I, Uzonna J (2009) Vaccines and vaccination strategies against human cutaneous leishmaniasis. Hum Vaccin 5: 291–230. - PubMed
-
- Requena JM, Alonso C, Soto M (2000) Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol Today 16: 246–250. - PubMed
-
- Galanti N, Galindo M, Sabaj V, Espinoza I, Toro GC (1998) Histone genes in trypanosomatids. Parasitol Today 14: 64–70. - PubMed

