C. elegans expressing human β2-microglobulin: a novel model for studying the relationship between the molecular assembly and the toxic phenotype
- PMID: 23284985
- PMCID: PMC3528749
- DOI: 10.1371/journal.pone.0052314
C. elegans expressing human β2-microglobulin: a novel model for studying the relationship between the molecular assembly and the toxic phenotype
Abstract
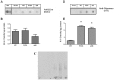
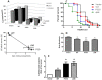
Availability of living organisms to mimic key step of amyloidogenesis of human protein has become an indispensable tool for our translation approach aiming at filling the deep gap existing between the biophysical and biochemical data obtained in vitro and the pathological features observed in patients. Human β(2)-microglobulin (β(2)-m) causes systemic amyloidosis in haemodialysed patients. The structure, misfolding propensity, kinetics of fibrillogenesis and cytotoxicity of this protein, in vitro, have been studied more extensively than for any other globular protein. However, no suitable animal model for β(2)-m amyloidosis has been so far reported. We have now established and characterized three new transgenic C. elegans strains expressing wild type human β(2)-m and two highly amyloidogenic isoforms: P32G variant and the truncated form ΔN6 lacking of the 6 N-terminal residues. The expression of human β(2)-m affects the larval growth of C. elegans and the severity of the damage correlates with the intrinsic propensity to self-aggregate that has been reported in previous in vitro studies. We have no evidence of the formation of amyloid deposits in the body-wall muscles of worms. However, we discovered a strict correlation between the pathological phenotype and the presence of oligomeric species recognized by the A11 antibody. The strains expressing human β(2)-m exhibit a locomotory defect quantified with the body bends assay. Here we show that tetracyclines can correct this abnormality confirming that these compounds are able to protect a living organism from the proteotoxicity of human β(2)-m.
Conflict of interest statement
Figures



References
-
- Link CD (2005) Invertebrate models of Alzheimer's disease. Genes Brain Behav 4: 147–156. - PubMed
-
- Lakso M, Vartiainen S, Moilanen AM, Sirviö J, Thomas JH, et al. (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem 86: 165–172. - PubMed
-
- Luo Y, Wu Y, Brown M, Link CD (2009) Caenorhabditis elegans Model for Initial Screening and Mechanistic Evaluation of Potential New Drugs for Aging and Alzheimer's Disease. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press; Chapter 16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

