Microparticle-mediated transfer of the viral receptors CAR and CD46, and the CFTR channel in a CHO cell model confers new functions to target cells
- PMID: 23284987
- PMCID: PMC3527531
- DOI: 10.1371/journal.pone.0052326
Microparticle-mediated transfer of the viral receptors CAR and CD46, and the CFTR channel in a CHO cell model confers new functions to target cells
Abstract
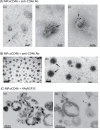
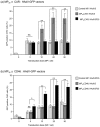
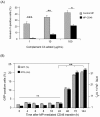
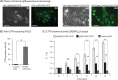
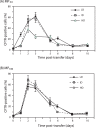
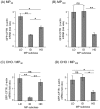
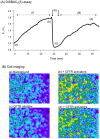
Cell microparticles (MPs) released in the extracellular milieu can embark plasma membrane and intracellular components which are specific of their cellular origin, and transfer them to target cells. The MP-mediated, cell-to-cell transfer of three human membrane glycoproteins of different degrees of complexity was investigated in the present study, using a CHO cell model system. We first tested the delivery of CAR and CD46, two monospanins which act as adenovirus receptors, to target CHO cells. CHO cells lack CAR and CD46, high affinity receptors for human adenovirus serotype 5 (HAdV5), and serotype 35 (HAdV35), respectively. We found that MPs derived from CHO cells (MP-donor cells) constitutively expressing CAR (MP-CAR) or CD46 (MP-CD46) were able to transfer CAR and CD46 to target CHO cells, and conferred selective permissiveness to HAdV5 and HAdV35. In addition, target CHO cells incubated with MP-CD46 acquired the CD46-associated function in complement regulation. We also explored the MP-mediated delivery of a dodecaspanin membrane glycoprotein, the CFTR to target CHO cells. CFTR functions as a chloride channel in human cells and is implicated in the genetic disease cystic fibrosis. Target CHO cells incubated with MPs produced by CHO cells constitutively expressing GFP-tagged CFTR (MP-GFP-CFTR) were found to gain a new cellular function, the chloride channel activity associated to CFTR. Time-course analysis of the appearance of GFP-CFTR in target cells suggested that MPs could achieve the delivery of CFTR to target cells via two mechanisms: the transfer of mature, membrane-inserted CFTR glycoprotein, and the transfer of CFTR-encoding mRNA. These results confirmed that cell-derived MPs represent a new class of promising therapeutic vehicles for the delivery of bioactive macromolecules, proteins or mRNAs, the latter exerting the desired therapeutic effect in target cells via de novo synthesis of their encoded proteins.
Conflict of interest statement
Figures


Similar articles
-
Ex Vivo and In Vivo CD46 Receptor Utilization by Species D Human Adenovirus Serotype 26 (HAdV26).J Virol. 2022 Feb 9;96(3):e0082621. doi: 10.1128/JVI.00826-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34787457 Free PMC article.
-
Transfer of the Cystic Fibrosis Transmembrane Conductance Regulator to Human Cystic Fibrosis Cells Mediated by Extracellular Vesicles.Hum Gene Ther. 2016 Feb;27(2):166-83. doi: 10.1089/hum.2015.144. Hum Gene Ther. 2016. PMID: 26886833
-
Targeting CD46 Enhances Anti-Tumoral Activity of Adenovirus Type 5 for Bladder Cancer.Int J Mol Sci. 2018 Sep 10;19(9):2694. doi: 10.3390/ijms19092694. Int J Mol Sci. 2018. PMID: 30201920 Free PMC article.
-
Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis.Semin Immunopathol. 2011 Sep;33(5):469-86. doi: 10.1007/s00281-010-0239-3. Epub 2011 Aug 25. Semin Immunopathol. 2011. PMID: 21866419 Review.
-
CD46 processing: a means of expression.Immunobiology. 2012 Feb;217(2):169-75. doi: 10.1016/j.imbio.2011.06.003. Epub 2011 Jul 13. Immunobiology. 2012. PMID: 21742405 Free PMC article. Review.
Cited by
-
Treatment of Cystic Fibrosis: From Gene- to Cell-Based Therapies.Front Pharmacol. 2021 Mar 16;12:639475. doi: 10.3389/fphar.2021.639475. eCollection 2021. Front Pharmacol. 2021. PMID: 33796025 Free PMC article. Review.
-
Microvesicles secreted from human multiple myeloma cells promote angiogenesis.Acta Pharmacol Sin. 2014 Feb;35(2):230-8. doi: 10.1038/aps.2013.141. Epub 2013 Dec 30. Acta Pharmacol Sin. 2014. PMID: 24374814 Free PMC article.
-
Advanced genetic engineering to achieve in vivo targeting of adenovirus utilizing camelid single domain antibody.J Control Release. 2021 Jun 10;334:106-113. doi: 10.1016/j.jconrel.2021.04.009. Epub 2021 Apr 16. J Control Release. 2021. PMID: 33872627 Free PMC article.
-
Endothelial Extracellular Vesicles in Pulmonary Function and Disease.Curr Top Membr. 2018;82:197-256. doi: 10.1016/bs.ctm.2018.09.002. Epub 2018 Oct 8. Curr Top Membr. 2018. PMID: 30360780 Free PMC article.
-
Decoding the role of extracellular vesicles in pathogenesis of cystic fibrosis.Mol Cell Pediatr. 2025 Apr 21;12(1):5. doi: 10.1186/s40348-025-00190-4. Mol Cell Pediatr. 2025. PMID: 40257719 Free PMC article. Review.
References
-
- Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907–1920. - PubMed
-
- Mause SF, Weber C (2010) Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057. - PubMed
-
- Stępień E, Kabłak-Ziembicka A, Czyż J, Przewłocki T, Małecki M (2012) Microparticles, not only markers but also a therapeutic target in the early stage of diabetic retinopathy and vascular aging. Expert Opin Ther Targets 16: 677–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

