Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response
- PMID: 23284990
- PMCID: PMC3528780
- DOI: 10.1371/journal.pone.0052330
Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response
Abstract
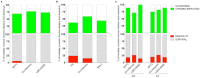
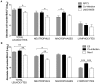
The Gram-negative bacteria Pseudomonas aeruginosa and Burkholderia cenocepacia are opportunistic human pathogens that are responsible for severe nosocomial infections in immunocompromised patients and those suffering from cystic fibrosis (CF). These two bacteria have been shown to form biofilms in the airways of CF patients that make such infections more difficult to treat. Only recently have scientists begun to appreciate the complicated interplay between microorganisms during polymicrobial infection of the CF airway and the implications they may have for disease prognosis and response to therapy.To gain insight into the possible role that interaction between strains of P. aeruginosa and B. cenocepacia may play during infection, we characterised co-inoculations of in vivo and in vitro infection models. Co-inoculations were examined in an in vitro biofilm model and in a murine model of chronic infection. Assessment of biofilm formation showed that B. cenocepacia positively influenced P. aeruginosa biofilm development by increasing biomass. Interestingly, co-infection experiments in the mouse model revealed that P. aeruginosa did not change its ability to establish chronic infection in the presence of B. cenocepacia but co-infection did appear to increase host inflammatory response.Taken together, these results indicate that the co-infection of P. aeruginosa and B. cenocepacia leads to increased biofilm formation and increased host inflammatory response in the mouse model of chronic infection. These observations suggest that alteration of bacterial behavior due to interspecies interactions may be important for disease progression and persistent infection.
Conflict of interest statement
Figures
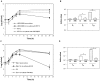
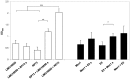
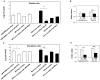
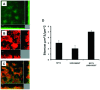
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

