Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway
- PMID: 23285052
- PMCID: PMC3527492
- DOI: 10.1371/journal.pone.0052465
Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway
Abstract
Background: Mesenchymal stem cells (MSCs) promote tumor growth by differentiating into carcinoma-associated fibroblasts (CAFs) and composing the tumor microenvironment. However, the mechanisms responsible for the transition of MSCs to CAFs are not well understood. Exosomes regulate cellular activities by mediating cell-cell communication. In this study, we aimed to investigate whether cancer cell-derived exosomes were involved in regulating the differentiation of human umbilical cord-derived MSCs (hucMSCs) to CAFs.
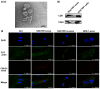
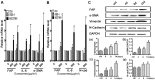
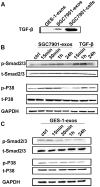
Methodology/principal findings: We first showed that gastric cancer cell-derived exosomes induced the expression of CAF markers in hucMSCs. We then demonstrated that gastric cancer cell-derived exosomes stimulated the phosphorylation of Smad-2 in hucMSCs. We further confirmed that TGF-β receptor 1 kinase inhibitor attenuated Smad-2 phosphorylation and CAF marker expression in hucMSCs after exposure to gastric cancer cell-derived exosomes.
Conclusion/significance: Our results suggest that gastric cancer cells triggered the differentiation of hucMSCs to CAFs by exosomes-mediated TGF-β transfer and TGF-β/Smad pathway activation, which may represent a novel mechanism for MSCs to CAFs transition in cancer.
Conflict of interest statement
Figures



References
-
- Joyce JA (2005) Therapeutic targeting of the tumor microenvironment. Cancer Cell 7: 513–520. - PubMed
-
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, et al. (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563. - PubMed
-
- Cao H, Xu W, Qian H, Zhu W, Yan Y, et al. (2009) Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett 274: 61–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

