N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1
- PMID: 23285087
- PMCID: PMC3527552
- DOI: 10.1371/journal.pone.0052563
N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1
Abstract
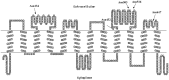
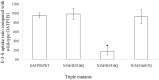
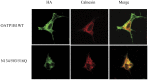
Organic anion transporting polypeptides (OATPs) have been extensively recognized as key determinants of absorption, distribution, metabolism and excretion (ADME) of various drugs, xenobiotics and toxins. Putative N-glycosylation sites located in the extracellular loops 2 and 5 is considered a common feature of all OATPs and some members have been demonstrated to be glycosylated proteins. However, experimental evidence is still lacking on how such a post-translational modification affect the transport activity of OATPs and which of the putative glycosylation sites are utilized in these transporter proteins. In the present study, we substituted asparagine residues that are possibly involved in N-glycosylation with glutamine residues and identified three glycosylation sites (Asn134, Asn503 and Asn516) within the structure of OATP1B1, an OATP member that is mainly expressed in the human liver. Our results showed that Asn134 and Asn516 are used for glycosylation under normal conditions; however, when Asn134 was mutagenized, an additional asparagine at position 503 is involved in the glycosylation process. Simultaneously replacement of all three asparagines with glutamines led to significantly reduced protein level as well as loss of transport activity. Further studies revealed that glycosylation affected stability of the transporter protein and the unglycosylated mutant was retained within endoplasmic reticulum.
Conflict of interest statement
Figures



Similar articles
-
Conserved tryptophan residues within putative transmembrane domain 6 affect transport function of organic anion transporting polypeptide 1B1.Mol Pharmacol. 2013 Oct;84(4):521-7. doi: 10.1124/mol.113.085977. Epub 2013 Jul 15. Mol Pharmacol. 2013. PMID: 23858103
-
Amino Acid Residues in the Putative Transmembrane Domain 11 of Human Organic Anion Transporting Polypeptide 1B1 Dictate Transporter Substrate Binding, Stability, and Trafficking.Mol Pharm. 2015 Dec 7;12(12):4270-6. doi: 10.1021/acs.molpharmaceut.5b00466. Epub 2015 Nov 20. Mol Pharm. 2015. PMID: 26562723
-
Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver.J Pharm Pharm Sci. 2014;17(4):475-84. doi: 10.18433/j3m89q. J Pharm Pharm Sci. 2014. PMID: 25579430
-
Critical domains within the sequence of human organic anion transporting polypeptides.Curr Drug Metab. 2014 Mar;15(3):265-70. doi: 10.2174/1389200214666131229111118. Curr Drug Metab. 2014. PMID: 24372098 Review.
-
Post-translational regulation of the major drug transporters in the families of organic anion transporters and organic anion-transporting polypeptides.J Biol Chem. 2020 Dec 11;295(50):17349-17364. doi: 10.1074/jbc.REV120.009132. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051208 Free PMC article. Review.
Cited by
-
Importance of N-Glycosylation for the Expression and Function of Human Organic Anion Transporting Polypeptide 2B1.ACS Pharmacol Transl Sci. 2023 Sep 1;6(10):1347-1356. doi: 10.1021/acsptsci.3c00076. eCollection 2023 Oct 13. ACS Pharmacol Transl Sci. 2023. PMID: 37854627 Free PMC article.
-
Characterization of SLCO5A1/OATP5A1, a solute carrier transport protein with non-classical function.PLoS One. 2013 Dec 20;8(12):e83257. doi: 10.1371/journal.pone.0083257. eCollection 2013. PLoS One. 2013. PMID: 24376674 Free PMC article.
-
Innovative approaches and recent advances in the study of ontogeny of drug metabolism and transport.Br J Clin Pharmacol. 2022 Oct;88(10):4285-4296. doi: 10.1111/bcp.14534. Epub 2020 Sep 15. Br J Clin Pharmacol. 2022. PMID: 32851677 Free PMC article. Review.
-
Impaired N-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease.Liver Int. 2017 Jul;37(7):1074-1081. doi: 10.1111/liv.13362. Epub 2017 Feb 7. Liver Int. 2017. PMID: 28097795 Free PMC article.
-
Loops and layers of post-translational modifications of drug transporters.Adv Drug Deliv Rev. 2017 Jul 1;116:37-44. doi: 10.1016/j.addr.2016.05.003. Epub 2016 May 9. Adv Drug Deliv Rev. 2017. PMID: 27174152 Free PMC article. Review.
References
-
- Hagenbuch B, Gui C (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38: 778–801. - PubMed
-
- Shitara Y, Sato H, Sugiyama Y (2005) Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol 45: 689–723. - PubMed
-
- Poirier A, Funk C, Lavé T, Noé J (2007) New strategies to address drug-drug interactions involving OATPs. Curr Opin Drug Discov Dev 10: 74–83. - PubMed
-
- Hagenbuch B, Meier PJ (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609: 1–18. - PubMed
-
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T (2004) The organic anion transporter (OATP) family. Drug Metab Pharmacokinet 19: 171–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

