N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1
- PMID: 23285087
- PMCID: PMC3527552
- DOI: 10.1371/journal.pone.0052563
N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1
Abstract
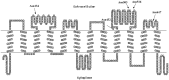
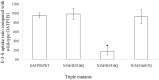
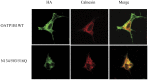
Organic anion transporting polypeptides (OATPs) have been extensively recognized as key determinants of absorption, distribution, metabolism and excretion (ADME) of various drugs, xenobiotics and toxins. Putative N-glycosylation sites located in the extracellular loops 2 and 5 is considered a common feature of all OATPs and some members have been demonstrated to be glycosylated proteins. However, experimental evidence is still lacking on how such a post-translational modification affect the transport activity of OATPs and which of the putative glycosylation sites are utilized in these transporter proteins. In the present study, we substituted asparagine residues that are possibly involved in N-glycosylation with glutamine residues and identified three glycosylation sites (Asn134, Asn503 and Asn516) within the structure of OATP1B1, an OATP member that is mainly expressed in the human liver. Our results showed that Asn134 and Asn516 are used for glycosylation under normal conditions; however, when Asn134 was mutagenized, an additional asparagine at position 503 is involved in the glycosylation process. Simultaneously replacement of all three asparagines with glutamines led to significantly reduced protein level as well as loss of transport activity. Further studies revealed that glycosylation affected stability of the transporter protein and the unglycosylated mutant was retained within endoplasmic reticulum.
Conflict of interest statement
Figures



References
-
- Hagenbuch B, Gui C (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38: 778–801. - PubMed
-
- Shitara Y, Sato H, Sugiyama Y (2005) Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol 45: 689–723. - PubMed
-
- Poirier A, Funk C, Lavé T, Noé J (2007) New strategies to address drug-drug interactions involving OATPs. Curr Opin Drug Discov Dev 10: 74–83. - PubMed
-
- Hagenbuch B, Meier PJ (2003) The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609: 1–18. - PubMed
-
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T (2004) The organic anion transporter (OATP) family. Drug Metab Pharmacokinet 19: 171–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

