ESCRT-independent budding of HIV-1 gag virus-like particles from Saccharomyces cerevisiae spheroplasts
- PMID: 23285107
- PMCID: PMC3528670
- DOI: 10.1371/journal.pone.0052603
ESCRT-independent budding of HIV-1 gag virus-like particles from Saccharomyces cerevisiae spheroplasts
Abstract
Heterologous expression of HIV-1 Gag in a variety of host cells results in its packaging into virus-like particles (VLPs) that are subsequently released into the extracellular milieu. This phenomenon represents a useful tool for probing cellular factors required for viral budding and has contributed to the discovery of roles for ubiquitin ligases and the endosomal sorting complexes required for transport (ESCRTs) in viral budding. These factors are highly conserved throughout eukaryotes and have been studied extensively in the yeast Saccharomyces cerevisiae, a model eukaryote previously utilized as a host for the production of VLPs. We used heterologous expression of HIV Gag in yeast spheroplasts to examine the role of ESCRTs and associated factors (Rsp5, a HECT ubiquitin ligase of the Nedd4 family; Bro1, a homolog of Alix; and Vps4, the AAA-ATPase required for ESCRT function in all contexts/organisms investigated) in the generation of VLPs. Our data reveal: 1) characterized Gag-ESCRT interaction motifs (late domains) are not required for VLP budding, 2) loss of function alleles of the essential HECT ubiquitin ligase Rsp5 do not display defects in VLP formation, and 3) ESCRT function is not required for VLP formation from spheroplasts. These results suggest that the egress of HIV Gag from yeast cells is distinct from the most commonly described mode of exit from mammalian cells, instead mimicking ESCRT-independent VLP formation observed in a subset of mammalian cells. As such, budding of Gag from yeast cells appears to represent ESCRT-independent budding relevant to viral replication in at least some situations. Thus the myriad of genetic and biochemical tools available in the yeast system may be of utility in the study of this aspect of viral budding.
Conflict of interest statement
Figures
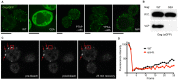
 ) expressing the indicated form of Gag-GFP were visualized by fluorescence microscopy. Scale bar for each image represents 4
) expressing the indicated form of Gag-GFP were visualized by fluorescence microscopy. Scale bar for each image represents 4  . (B) Yeast cells expressing either WT Gag-GFP or
. (B) Yeast cells expressing either WT Gag-GFP or  were analyzed for their ability to incorporate these chimeras into VLPs. Whole cell extracts (W.C.) and the VLP fractions were subjected to SDS-PAGE and western blotting with anti-GFP antibody to allow visualization. (C) Representative images of fluorescence recovery after photobleaching performed on wild type cells expressing Gag-GFP (pre-bleach, first image captured post-bleach, and 25 minute recovery). The red box highlights a punctum that was subjected to bleaching while the red arrows highlight Gag-GFP puncta that were not. (D) Quantification of fluorescence recovery after photobleaching (at one frame per minute) for wild type and
were analyzed for their ability to incorporate these chimeras into VLPs. Whole cell extracts (W.C.) and the VLP fractions were subjected to SDS-PAGE and western blotting with anti-GFP antibody to allow visualization. (C) Representative images of fluorescence recovery after photobleaching performed on wild type cells expressing Gag-GFP (pre-bleach, first image captured post-bleach, and 25 minute recovery). The red box highlights a punctum that was subjected to bleaching while the red arrows highlight Gag-GFP puncta that were not. (D) Quantification of fluorescence recovery after photobleaching (at one frame per minute) for wild type and  cells expressing wild type Gag-GFP.
cells expressing wild type Gag-GFP.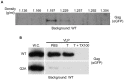
 (G2A) were equivalently processed to isolate the whole cell and VLP fractions. The VLP fraction was subsequently incubated with either PBS alone (PBS), PBS+Trypsin (T), or PBS+Trypsin+Triton X-100 (T+TX100), and post reaction material was visualized by immunoblotting using anti-GFP antibody. Whole cell lysates (W.C.) from cells expressing either WT Gag-GFP or
(G2A) were equivalently processed to isolate the whole cell and VLP fractions. The VLP fraction was subsequently incubated with either PBS alone (PBS), PBS+Trypsin (T), or PBS+Trypsin+Triton X-100 (T+TX100), and post reaction material was visualized by immunoblotting using anti-GFP antibody. Whole cell lysates (W.C.) from cells expressing either WT Gag-GFP or  used to generate the VLP fraction were loaded onto the gel to indicate the relative amount of expression.
used to generate the VLP fraction were loaded onto the gel to indicate the relative amount of expression.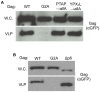
 ,
,  ,
,  ,
,  or
or  . VLP budding of the Gag-GFP constructs was assayed as described in the Methods section. Gag-GFP was visualized by Western blotting of whole cell extracts and VLP fractions with anti-GFP antibody.
. VLP budding of the Gag-GFP constructs was assayed as described in the Methods section. Gag-GFP was visualized by Western blotting of whole cell extracts and VLP fractions with anti-GFP antibody.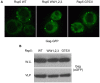
 was transformed into an
was transformed into an  background complemented with
background complemented with  ,
,  , or
, or  . (A) Localization of
. (A) Localization of  by fluorescence microscopy on the indicated live cells. (B) VLP budding assays were conducted with cells of the indicated genetic backgrounds, and whole cell extracts (W.C.) and VLP fractions were visualized by anti-GFP immunoblotting.
by fluorescence microscopy on the indicated live cells. (B) VLP budding assays were conducted with cells of the indicated genetic backgrounds, and whole cell extracts (W.C.) and VLP fractions were visualized by anti-GFP immunoblotting.
 or GFP-Cps1 were transformed into wild type cells or strains deleted for a component of each ESCRT complex, ESCRT-0 (
or GFP-Cps1 were transformed into wild type cells or strains deleted for a component of each ESCRT complex, ESCRT-0 ( ), ESCRT-I (
), ESCRT-I ( ), ESCRT-II (
), ESCRT-II ( ), ESCRT-III (
), ESCRT-III ( ) or VPS4 (
) or VPS4 ( ), and GFP localization was assessed by fluorescence microscopy of live cells. In the wild type background GFP-Cps1 was sorted into the lumen of the yeast vacuole, while loss of ESCRT function, Bro1 function, or Vps4 function resulted in missorting to the vacuolar limiting membrane and accumulation within the aberrant class E compartment. (B)
), and GFP localization was assessed by fluorescence microscopy of live cells. In the wild type background GFP-Cps1 was sorted into the lumen of the yeast vacuole, while loss of ESCRT function, Bro1 function, or Vps4 function resulted in missorting to the vacuolar limiting membrane and accumulation within the aberrant class E compartment. (B)  or
or  were expressed in the indicated strains strains and whole cell extracts (W.C.) and VLP fractions were analyzed by Western blotting with anti-GFP antibody. (C) VLPs were collected from
were expressed in the indicated strains strains and whole cell extracts (W.C.) and VLP fractions were analyzed by Western blotting with anti-GFP antibody. (C) VLPs were collected from  cells expressing wild type Gag-GFP and subjected to centrifugation across a sucrose gradient (20–70%, weight to volume). 1 ml fractions were subsequently collected, the density of each fraction was measured, and each fraction was subjected to SDS-PAGE and anti-GFP immunoblotting. (D)
cells expressing wild type Gag-GFP and subjected to centrifugation across a sucrose gradient (20–70%, weight to volume). 1 ml fractions were subsequently collected, the density of each fraction was measured, and each fraction was subjected to SDS-PAGE and anti-GFP immunoblotting. (D)  cells expressing wild type Gag-GFP were processed to isolate the whole cell and VLP fractions. The VLP fraction was subsequently subjected to incubation with either PBS alone (PBS), PBS+Trypsin (T), or PBS+Trypsin+Triton X-100 (T+TX100) and the reactions were visualized by immunoblotting using anti-GFP antibody. Whole cell lysate (W.C.) from the cells used to generate the VLP fraction was loaded to indicate expression.
cells expressing wild type Gag-GFP were processed to isolate the whole cell and VLP fractions. The VLP fraction was subsequently subjected to incubation with either PBS alone (PBS), PBS+Trypsin (T), or PBS+Trypsin+Triton X-100 (T+TX100) and the reactions were visualized by immunoblotting using anti-GFP antibody. Whole cell lysate (W.C.) from the cells used to generate the VLP fraction was loaded to indicate expression.Similar articles
-
HIV-1 Gag release from yeast reveals ESCRT interaction with the Gag N-terminal protein region.J Biol Chem. 2020 Dec 25;295(52):17950-17972. doi: 10.1074/jbc.RA120.014710. Epub 2020 Sep 28. J Biol Chem. 2020. PMID: 32994219 Free PMC article.
-
Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding.Retrovirology. 2013 Jul 29;10:79. doi: 10.1186/1742-4690-10-79. Retrovirology. 2013. PMID: 23895345 Free PMC article.
-
Rescue of HIV-1 release by targeting widely divergent NEDD4-type ubiquitin ligases and isolated catalytic HECT domains to Gag.PLoS Pathog. 2010 Sep 16;6(9):e1001107. doi: 10.1371/journal.ppat.1001107. PLoS Pathog. 2010. PMID: 20862313 Free PMC article.
-
The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview.Int J Biol Macromol. 2019 Apr 15;127:1-11. doi: 10.1016/j.ijbiomac.2019.01.015. Epub 2019 Jan 4. Int J Biol Macromol. 2019. PMID: 30615963 Review.
-
Budding of a Retrovirus: Some Assemblies Required.Viruses. 2020 Oct 20;12(10):1188. doi: 10.3390/v12101188. Viruses. 2020. PMID: 33092109 Free PMC article. Review.
Cited by
-
The Race against Protease Activation Defines the Role of ESCRTs in HIV Budding.PLoS Pathog. 2016 Jun 9;12(6):e1005657. doi: 10.1371/journal.ppat.1005657. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27280284 Free PMC article.
-
Gaussian curvature and the budding kinetics of enveloped viruses.PLoS Comput Biol. 2019 Aug 21;15(8):e1006602. doi: 10.1371/journal.pcbi.1006602. eCollection 2019 Aug. PLoS Comput Biol. 2019. PMID: 31433804 Free PMC article.
-
Inside job: how the ESCRTs release HIV-1 from infected cells.Biochem Soc Trans. 2018 Oct 19;46(5):1029-1036. doi: 10.1042/BST20180019. Epub 2018 Aug 28. Biochem Soc Trans. 2018. PMID: 30154094 Free PMC article. Review.
-
Exacerbating and reversing lysosomal storage diseases: from yeast to humans.Microb Cell. 2017 Aug 25;4(9):278-293. doi: 10.15698/mic2017.09.588. Microb Cell. 2017. PMID: 28913343 Free PMC article. Review.
-
ESCRT-II functions by linking to ESCRT-I in human immunodeficiency virus-1 budding.Cell Microbiol. 2020 May;22(5):e13161. doi: 10.1111/cmi.13161. Epub 2020 Feb 14. Cell Microbiol. 2020. PMID: 31922351 Free PMC article.
References
-
- Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD (1982) Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 298: 347–350. - PubMed
-
- Janda M, Ahlquist P (1993) RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell 72: 961–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

