Repair of abdominal wall defects with biodegradable laminar prostheses: polymeric or biological?
- PMID: 23285119
- PMCID: PMC3528658
- DOI: 10.1371/journal.pone.0052628
Repair of abdominal wall defects with biodegradable laminar prostheses: polymeric or biological?
Abstract
Introduction: Biological and synthetic laminar absorbable prostheses are available for the repair of hernia defects in the abdominal wall. They share the important feature of being gradually degraded in the host, resulting in place the formation of a neotissue. This study was designed to assess the host tissue's incorporation of collagen bioprostheses and a synthetic absorbable prosthesis.
Methods: Partial defects were created in the abdominal walls of 72 New Zealand rabbits and repaired using collagen bioprostheses Tutomesh® and Strattice® or a synthetic prosthesis Bio-A®. Specimens were collected for light microscopy, collagens gene and protein expression, macrophage response and biomechanical resistance at 14, 30, 90 and 180 days post-implantation.
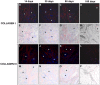
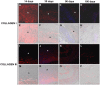
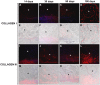
Results: Tutomesh® and Bio-A® were gradually infiltrated by the host tissue and almost completely degraded by 180 days post-implantation. In contrast, Strattice® exhibited material encapsulation, no prosthetic degradation and low cell infiltration at earlier timepoints, whereas at later study time, collagen deposition could be observed within the mesh. In the short term, Bio-A® exhibited higher level of collagen 1 and 3 mRNA expression compared with the two other biological prostheses, which exhibited two peaks of higher expression at 14 and 90 days. The expression of collagen III was homogeneous throughout the study and collagen I deposition was more evident in Strattice®. Macrophage response decreased over time in biomeshes. However, in the synthetic mesh remained high and homogeneous until 90 days. The biomechanical analysis demonstrated the progressively increasing tensile strength of all biomaterials.
Conclusions: The tissue infiltration of laminar absorbable prostheses is affected by the structure and composition of the mesh. The synthetic prosthesis exhibited a distinct pattern of tissue incorporation and a greater macrophage response than did the biological prostheses. Of all of the laminar, absorbable biomaterials that were tested in this study, Strattice® demonstrated the optimal levels of integration and degradation.
Conflict of interest statement
Figures






References
-
- Bellows CF, Albo D, Berger DH, Awad SS (2007) Abdominal wall repair using human acellular dermis. Am J Surg 194: 192–198. - PubMed
-
- Liang HCh, Chang Y, Hsu ChK, Lee MH, Sung HW (2004) Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 25: 3541–3552. - PubMed
-
- Losanoff JE, Richman BW, Jones JW (2002) Entero-colocutaneous fistula: a late consequence of polypropylene mesh abdominal wall repair: case report and review of the literature. Hernia 6: 144–147. - PubMed
-
- Petter-Puchner AH, Fortelny RH, Walder N, Mittermayr R, Öhlinger W, et al. (2008) Adverse effects associated with the use of porcine cross-linked collagen implants in an experimental model of incisional hernia repair. J Surg Res 145: 105–110. - PubMed
-
- Harth KC, Rosen MJ (2009) Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innnov 16: 324–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

