Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells
- PMID: 23285157
- PMCID: PMC3532360
- DOI: 10.1371/journal.pone.0052700
Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells
Abstract
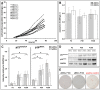
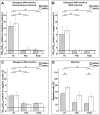
Mesenchymal stromal cells (MSCs) are of high relevance for the regeneration of mesenchymal tissues such as bone and cartilage. The promising role of MSCs in cell-based therapies and tissue engineering appears to be limited due to a decline of their regenerative potential with increasing donor age, their limited availability in human tissues and the need of in vitro expansion prior to treatment. We therefore aimed to determine to which degree in vitro aging and chronological aging may be similar processes or if in vitro culture-related changes at the cellular and molecular level are at least altered as a function of donor age. For that purpose we established MSCs cultures from young (yMSCs) and aged (aMSCs) rats that were cultured for more than 100 passages. These long-term MSCs cultures were non-tumorigenic and exhibited similar surface marker patterns as primary MSCs of passage 2. During in vitro expansion, but not during chronological aging, MSCs progressively lose their progenitor characteristics, e.g., complete loss of osteogenic differentiation potential, diminished adipogenic differentiation, altered cell morphology and increased susceptibility towards senescence. Transcriptome analysis revealed that long-term in vitro MSCs cultivation leads to down-regulation of genes involved in cell differentiation, focal adhesion organization, cytoskeleton turnover and mitochondria function. Accordingly, functional analysis demonstrated altered mitochondrial morphology, decreased antioxidant capacities and elevated ROS levels in long-term cultivated yMSCs as well as aMSCs. Notably, only the MSC migration potential and their antioxidative capacity were altered by in vitro as well as chronological aging. Based on specific differences observed between the impact of chronological and in vitro MSC aging we conclude that both are distinct processes.
Conflict of interest statement
Figures



References
-
- Kasper G, Glaeser JD, Geissler S, Ode A, Tuischer J, et al. (2007) Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 25: 1985–1994. - PubMed
-
- Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, et al. (2007) Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells 25: 903–910. - PubMed
-
- Krampera M, Pizzolo G, Aprili G, Franchini M (2006) Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 39: 678–683. - PubMed
-
- Le Blanc K, Pittenger M (2005) Mesenchymal stem cells: progress toward promise. Cytotherapy 7: 36–45. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

