Optimized Pan-species and speciation duplex real-time PCR assays for Plasmodium parasites detection in malaria vectors
- PMID: 23285168
- PMCID: PMC3532469
- DOI: 10.1371/journal.pone.0052719
Optimized Pan-species and speciation duplex real-time PCR assays for Plasmodium parasites detection in malaria vectors
Abstract
Background: An accurate method for detecting malaria parasites in the mosquito's vector remains an essential component in the vector control. The Enzyme linked immunosorbent assay specific for circumsporozoite protein (ELISA-CSP) is the gold standard method for the detection of malaria parasites in the vector even if it presents some limitations. Here, we optimized multiplex real-time PCR assays to accurately detect minor populations in mixed infection with multiple Plasmodium species in the African malaria vectors Anopheles gambiae and Anopheles funestus.
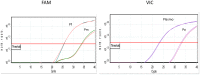
Methods: Complementary TaqMan-based real-time PCR assays that detect Plasmodium species using specific primers and probes were first evaluated on artificial mixtures of different targets inserted in plasmid constructs. The assays were further validated in comparison with the ELISA-CSP on 200 field caught Anopheles gambiae and Anopheles funestus mosquitoes collected in two localities in southern Benin.
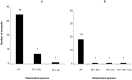
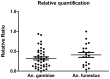
Results: The validation of the duplex real-time PCR assays on the plasmid mixtures demonstrated robust specificity and sensitivity for detecting distinct targets. Using a panel of mosquito specimen, the real-time PCR showed a relatively high sensitivity (88.6%) and specificity (98%), compared to ELISA-CSP as the referent standard. The agreement between both methods was "excellent" (κ=0.8, P<0.05). The relative quantification of Plasmodium DNA between the two Anopheles species analyzed showed no significant difference (P=0, 2). All infected mosquito samples contained Plasmodium falciparum DNA and mixed infections with P. malariae and/or P. ovale were observed in 18.6% and 13.6% of An. gambiae and An. funestus respectively. Plasmodium vivax was found in none of the mosquito samples analyzed.
Conclusion: This study presents an optimized method for detecting the four Plasmodium species in the African malaria vectors. The study highlights substantial discordance with traditional ELISA-CSP pointing out the utility of employing an accurate molecular diagnostic tool for detecting malaria parasites in field mosquito populations.
Conflict of interest statement
Figures
Similar articles
-
Semi-high-throughput detection of Plasmodium falciparum and Plasmodium vivax oocysts in mosquitoes using bead-beating followed by circumsporozoite ELISA and quantitative PCR.Malar J. 2017 Sep 6;16(1):356. doi: 10.1186/s12936-017-2011-9. Malar J. 2017. PMID: 28877707 Free PMC article.
-
Implementation of a novel PCR based method for detecting malaria parasites from naturally infected mosquitoes in Papua New Guinea.Malar J. 2009 Aug 1;8:182. doi: 10.1186/1475-2875-8-182. Malar J. 2009. PMID: 19646275 Free PMC article.
-
Evaluation of a real-time quantitative PCR to measure the wild Plasmodium falciparum infectivity rate in salivary glands of Anopheles gambiae.Malar J. 2013 Jul 2;12:224. doi: 10.1186/1475-2875-12-224. Malar J. 2013. PMID: 23819831 Free PMC article.
-
[The utilization of molecular biological tools in the study of malaria transmission: example of programs conducted in Senegal].Med Trop (Mars). 1995;55(4 Suppl):52-5. Med Trop (Mars). 1995. PMID: 8649267 Review. French.
-
An overview of malaria transmission from the perspective of Amazon Anopheles vectors.Mem Inst Oswaldo Cruz. 2015 Feb;110(1):23-47. doi: 10.1590/0074-02760140266. Epub 2015 Feb 13. Mem Inst Oswaldo Cruz. 2015. PMID: 25742262 Free PMC article. Review.
Cited by
-
A sensitive, specific and reproducible real-time polymerase chain reaction method for detection of Plasmodium vivax and Plasmodium falciparum infection in field-collected anophelines.Mem Inst Oswaldo Cruz. 2015 Jun;110(4):573-6. doi: 10.1590/0074-02760150031. Epub 2015 May 29. Mem Inst Oswaldo Cruz. 2015. PMID: 26061150 Free PMC article.
-
Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates.PLoS One. 2013 May 21;8(5):e63849. doi: 10.1371/journal.pone.0063849. Print 2013. PLoS One. 2013. PMID: 23704944 Free PMC article.
-
Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar.Parasit Vectors. 2020 Aug 26;13(1):430. doi: 10.1186/s13071-020-04282-0. Parasit Vectors. 2020. PMID: 32843082 Free PMC article.
-
Assessing Plasmodium falciparum transmission in mosquito-feeding assays using quantitative PCR.Malar J. 2018 Jul 5;17(1):249. doi: 10.1186/s12936-018-2382-6. Malar J. 2018. PMID: 29976199 Free PMC article.
-
Mapping the distribution of Anopheles funestus across Benin highlights a sharp contrast of susceptibility to insecticides and infection rate to Plasmodium between southern and northern populations.Wellcome Open Res. 2016 Dec 14;1:28. doi: 10.12688/wellcomeopenres.10213.2. Wellcome Open Res. 2016. PMID: 28191507 Free PMC article.
References
-
- Organization WH (2011) World Malaria Report 2011. Geneva: World Health Organization and UNICEF.
-
- SNIGS (2010) Rapport d’activité 2010 de l’IRD au Bénin. 6P.
-
- World Health Organization and United Nations Children’s Fund (2005) World Malaria Report 2005.Geneva: World Health Organization and UNICEF.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

