Centaurin-α₂ interacts with β-tubulin and stabilizes microtubules
- PMID: 23285209
- PMCID: PMC3527619
- DOI: 10.1371/journal.pone.0052867
Centaurin-α₂ interacts with β-tubulin and stabilizes microtubules
Abstract
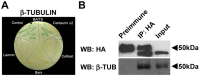
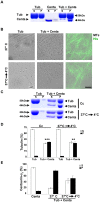
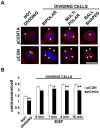
Centaurin-α₂ is a GTPase-activating protein for ARF (ARFGAP) showing a diffuse cytoplasmic localization capable to translocate to membrane, where it binds phosphatidylinositols. Taking into account that Centaurin-α₂ can localize in cytoplasm and that its cytoplasmatic function is not well defined, we searched for further interactors by yeast two-hybrid assay to investigate its biological function. We identified a further Centaurin-α₂ interacting protein, β-Tubulin, by yeast two-hybrid assay. The interaction, involving the C-terminal region of β-Tubulin, has been confirmed by coimmunoprecipitation experiments. After Centaurin-α₂ overexpression in HeLa cells and extraction of soluble (αβ dimers) and insoluble (microtubules) fractions of Tubulin, we observed that Centaurin-α₂ mainly interacts with the polymerized Tubulin fraction, besides colocalizing with microtubules (MTs) in cytoplasm accordingly. Even following the depolimerizing Tubulin treatments Centaurin-α₂ remains mainly associated to nocodazole- and cold-resistant MTs. We found an increase of MT stability in transfected HeLa cells, evaluating as marker of stability the level of MT acetylation. In vitro assays using purified Centaurin-α₂ and tubulin confirmed that Centaurin-α₂ promotes tubulin assembly and increases microtubule stability. The biological effect of Centaurin-α₂ overexpression, assessed through the detection of an increased number of mitotic HeLa cells with bipolar spindles and with the correct number of centrosomes in both dividing and not dividing cells, is consistent with the Centaurin-α₂ role on MT stabilization. Centaurin-α₂ interacts with β-Tubulin and it mainly associates to MTs, resistant to destabilizing agents, in vitro and in cell. We propose Centaurin-α₂ as a new microtubule-associated protein (MAP) increasing MT stability.
Conflict of interest statement
Figures



Similar articles
-
Cep70 regulates microtubule stability by interacting with HDAC6.FEBS Lett. 2015 Jul 8;589(15):1771-7. doi: 10.1016/j.febslet.2015.06.017. Epub 2015 Jun 22. FEBS Lett. 2015. PMID: 26112604
-
Furry promotes acetylation of microtubules in the mitotic spindle by inhibition of SIRT2 tubulin deacetylase.J Cell Sci. 2013 Oct 1;126(Pt 19):4369-80. doi: 10.1242/jcs.127209. Epub 2013 Jul 25. J Cell Sci. 2013. PMID: 23886946
-
Microtubule stabilization by Mdp3 is partially attributed to its modulation of HDAC6 in addition to its association with tubulin and microtubules.PLoS One. 2014 Mar 10;9(3):e90932. doi: 10.1371/journal.pone.0090932. eCollection 2014. PLoS One. 2014. PMID: 24614595 Free PMC article.
-
Microtubule acetylation dyshomeostasis in Parkinson's disease.Transl Neurodegener. 2023 May 8;12(1):20. doi: 10.1186/s40035-023-00354-0. Transl Neurodegener. 2023. PMID: 37150812 Free PMC article. Review.
-
[Microtubules: functional polymorphisms of tubulin and associated proteins (structural and motor MAP's)].C R Seances Soc Biol Fil. 1996;190(2-3):255-68. C R Seances Soc Biol Fil. 1996. PMID: 8869236 Review. French.
Cited by
-
Essential genes shape cancer genomes through linear limitation of homozygous deletions.Commun Biol. 2019 Jul 19;2:262. doi: 10.1038/s42003-019-0517-0. eCollection 2019. Commun Biol. 2019. PMID: 31341961 Free PMC article.
-
Emerging genotype-phenotype relationships in patients with large NF1 deletions.Hum Genet. 2017 Apr;136(4):349-376. doi: 10.1007/s00439-017-1766-y. Epub 2017 Feb 17. Hum Genet. 2017. PMID: 28213670 Free PMC article. Review.
-
Identification of an atypical microdeletion generating the RNF135-SUZ12 chimeric gene and causing a position effect in an NF1 patient with overgrowth.Hum Genet. 2017 Oct;136(10):1329-1339. doi: 10.1007/s00439-017-1832-5. Epub 2017 Aug 3. Hum Genet. 2017. PMID: 28776093
-
Aggresome formation is regulated by RanBPM through an interaction with HDAC6.Biol Open. 2014 May 2;3(6):418-30. doi: 10.1242/bio.20147021. Biol Open. 2014. PMID: 24795145 Free PMC article.
-
Identification of key genes as predictive biomarkers for osteosarcoma metastasis using translational bioinformatics.Cancer Cell Int. 2021 Dec 2;21(1):640. doi: 10.1186/s12935-021-02308-w. Cancer Cell Int. 2021. PMID: 34856991 Free PMC article.
References
-
- Cukierman E, Huber I, Rotman M, Cassel D (1995) The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270: 1999–2002. - PubMed
-
- Hanck T, Stricker R, Sedehizade F, Reiser G (2004) Identification of gene structure and subcellular localization of human centaurin alpha 2, and p42IP4, a family of two highly homologous, Ins 1,3,4,5-P4-/PtdIns 3,4,5-P3-binding, adapter proteins. J Neurochem 88: 326–336. - PubMed
-
- Venkateswarlu K, Brandom KG, Yun H (2007) PI-3-kinase-dependent membrane recruitment of centaurin-alpha2 is essential for its effect on ARF6-mediated actin cytoskeleton reorganisation. J Cell Sci 120: 792–801. - PubMed
-
- Venturin M, Bentivegna A, Moroni R. Larizza L, Riva P (2005) Evidence by expression analysis of candidate genes for congenital heart defects in the NF1 microdeletion interval. Ann Hum Genet 69: 508–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

