Human sensory neurons derived from induced pluripotent stem cells support varicella-zoster virus infection
- PMID: 23285249
- PMCID: PMC3532467
- DOI: 10.1371/journal.pone.0053010
Human sensory neurons derived from induced pluripotent stem cells support varicella-zoster virus infection
Abstract
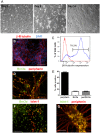
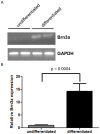
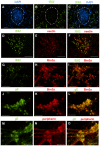
After primary infection, varicella-zoster virus (VZV) establishes latency in neurons of the dorsal root and trigeminal ganglia. Many questions concerning the mechanism of VZV pathogenesis remain unanswered, due in part to the strict host tropism and inconsistent availability of human tissue obtained from autopsies and abortions. The recent development of induced pluripotent stem (iPS) cells provides great potential for the study of many diseases. We previously generated human iPS cells from skin fibroblasts by introducing four reprogramming genes with non-integrating adenovirus. In this study, we developed a novel protocol to generate sensory neurons from iPS cells. Human iPS cells were exposed to small molecule inhibitors for 10 days, which efficiently converted pluripotent cells into neural progenitor cells (NPCs). The NPCs were then exposed for two weeks to growth factors required for their conversion to sensory neurons. The iPS cell-derived sensory neurons were characterized by immunocytochemistry, flow cytometry, RT-qPCR, and electrophysiology. After differentiation, approximately 80% of the total cell population expressed the neuron-specific protein, βIII-tubulin. Importantly, 15% of the total cell population co-expressed the markers Brn3a and peripherin, indicating that these cells are sensory neurons. These sensory neurons could be infected by both VZV and herpes simplex virus (HSV), a related alphaherpesvirus. Since limited neuronal populations are capable of supporting the entire VZV and HSV life cycles, our iPS-derived sensory neuron model may prove useful for studying alphaherpesvirus latency and reactivation.
Conflict of interest statement
Figures



Similar articles
-
Role of the JNK Pathway in Varicella-Zoster Virus Lytic Infection and Reactivation.J Virol. 2017 Aug 10;91(17):e00640-17. doi: 10.1128/JVI.00640-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637759 Free PMC article.
-
Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts In Vivo.PLoS Pathog. 2015 Jun 19;11(6):e1004989. doi: 10.1371/journal.ppat.1004989. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26090802 Free PMC article.
-
Differentiation of Human Neurons and Neuron-Like Cells to Study VZV Neuronal Infection.Methods Mol Biol. 2025;2950:87-105. doi: 10.1007/978-1-0716-4674-8_7. Methods Mol Biol. 2025. PMID: 40622530
-
Experimental models to study varicella-zoster virus infection of neurons.Curr Top Microbiol Immunol. 2010;342:211-28. doi: 10.1007/82_2010_15. Curr Top Microbiol Immunol. 2010. PMID: 20373093 Review.
-
Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation.Viruses. 2019 Jan 26;11(2):103. doi: 10.3390/v11020103. Viruses. 2019. PMID: 30691086 Free PMC article. Review.
Cited by
-
Connectivity and circuitry in a dish versus in a brain.Alzheimers Res Ther. 2015 Jun 4;7(1):44. doi: 10.1186/s13195-015-0129-y. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 26045718 Free PMC article.
-
An engineered three-dimensional stem cell niche in the inner ear by applying a nanofibrillar cellulose hydrogel with a sustained-release neurotrophic factor delivery system.Acta Biomater. 2020 May;108:111-127. doi: 10.1016/j.actbio.2020.03.007. Epub 2020 Mar 7. Acta Biomater. 2020. PMID: 32156626 Free PMC article.
-
Role of the JNK Pathway in Varicella-Zoster Virus Lytic Infection and Reactivation.J Virol. 2017 Aug 10;91(17):e00640-17. doi: 10.1128/JVI.00640-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637759 Free PMC article.
-
Modeling HSV-1 Latency in Human Embryonic Stem Cell-Derived Neurons.Pathogens. 2017 Jun 8;6(2):24. doi: 10.3390/pathogens6020024. Pathogens. 2017. PMID: 28594343 Free PMC article.
-
Compartmentalized Neuronal Culture for Viral Transport Research.Front Microbiol. 2020 Jul 15;11:1470. doi: 10.3389/fmicb.2020.01470. eCollection 2020. Front Microbiol. 2020. PMID: 32760359 Free PMC article. Review.
References
-
- Arvin AM (1992) Cell-mediated immunity to varicella-zoster virus. J Infect Dis 166 Suppl 1: S35–S41. - PubMed
-
- Arvin AM, Moffat JF, Redman R (1996) Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res 46: 263–309. - PubMed
-
- Oxman MN (2009) Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc 109: S13–S17 109/6_suppl_2/S13 [pii]. - PubMed
-
- Weinberg A, Levin MJ (2010) VZV T cell-mediated immunity. Curr Top Microbiol Immunol 342: 341–357 10.1007/82_2010_31 [doi]. - PubMed
-
- Dworkin RH, White R, O'Connor AB, Baser O, Hawkins K (2007) Healthcare costs of acute and chronic pain associated with a diagnosis of herpes zoster. J Am Geriatr Soc 55: 1168–1175 JGS1231 [pii];10.1111/j.1532-5415.2007.01231.x [doi]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

