Striatal cholinergic interneurons display activity-related phosphorylation of ribosomal protein S6
- PMID: 23285266
- PMCID: PMC3532298
- DOI: 10.1371/journal.pone.0053195
Striatal cholinergic interneurons display activity-related phosphorylation of ribosomal protein S6
Abstract
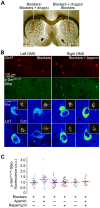
Cholinergic interneurons (CINs) provide the main source of acetylcholine to all striatal regions, and strongly modulate dopaminergic actions through complex regulation of pre- and post-synaptic acetylcholine receptors. Although striatal CINs have a well-defined electrophysiological profile, their biochemical properties are poorly understood, likely due to their low proportion within the striatum (2-3%). We report a strong and sustained phosphorylation of ribosomal protein S6 on its serine 240 and 244 residues (p-Ser²⁴⁰⁻²⁴⁴-S6rp), a protein integrant of the ribosomal machinery related to the mammalian target of the rapamycin complex 1 (mTORC1) pathway, which we found to be principally expressed in striatal CINs in basal conditions. We explored the functional relevance of this cellular event by pharmacologically inducing various sustained physiological activity states in CINs and assessing the effect on the levels of S6rp phosphorylation. Cell-attached electrophysiological recordings from CINs in a striatal slice preparation showed an inhibitory effect of tetrodotoxin (TTX) on action potential firing paralleled by a decrease in the p-Ser²⁴⁰⁻²⁴⁴-S6rp signal as detected by immunofluorescence after prolonged incubation. On the other hand, elevation in extracellular potassium concentration and the addition of apamin generated an increased firing rate and a burst-firing activity in CINs, respectively, and both stimulatory conditions significantly increased Ser²⁴⁰⁻²⁴⁴-S6rp phosphorylation above basal levels when incubated for one hour. Apamin generated a particularly large increase in phosphorylation that was sensitive to rapamycin. Taken together, our results demonstrate for the first time a link between the state of neuronal activity and a biochemical signaling event in striatal CINs, and suggest that immunofluorescence can be used to estimate the cellular activity of CINs under different pharmacological and/or behavioral conditions.
Conflict of interest statement
Figures





References
-
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience 9: 357–381. - PubMed
-
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends in neurosciences 18: 527–535. - PubMed
-
- Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Current opinion in neurobiology 14: 685–692. - PubMed
-
- Bolam JP (1984) Synapses of identified neurons in the neostriatum. Ciba Foundation symposium 107: 30–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

