Alveolar type II cells possess the capability of initiating lung tumor development
- PMID: 23285300
- PMCID: PMC3527621
- DOI: 10.1371/journal.pone.0053817
Alveolar type II cells possess the capability of initiating lung tumor development
Abstract
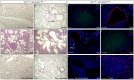
Identifying cells of tumor origin is a fundamental question in tumor biology. Answers to this central question will not only advance our understanding of tumor initiation and progression but also have important therapeutic implications. In this study, we aimed to uncover the cells of origin of lung adenocarcinoma, a major subtype of non-small cell lung cancer. To this end, we developed new mouse models of lung adenocarcinoma that enabled selective manipulation of gene activity in surfactant associated protein C (SPC)-expressing cells, including alveolar type II cells and bronchioalveolar stem cells (BASCs) that reside at the bronchioalveolar duct junction (BADJ). Our findings showed that activation of oncogenic Kras alone or in combination with the removal of the tumor suppressor p53 in SPC⁺ cells resulted in development of alveolar tumors. Similarly, sustained EGF signaling in SPC⁺ cells led to alveolar tumors. By contrast, BASCs failed to proliferate or produce tumors under these conditions. Importantly, in a mouse strain in which Kras/p53 activity was selectively altered in type II cells but not BASCs, alveolar tumors developed while BADJs retained normal architecture. These results confirm and extend previous findings and support a model in which lung adenocarcinoma can initiate in alveolar type II cells. Our results establish the foundation for elucidating the molecular mechanisms by which lung cancer initiates and progresses in a specific lung cell type.
Conflict of interest statement
Figures



References
-
- McErlean A, Ginsberg MS (2011) Epidemiology of lung cancer. Semin Roentgenol 46: 173–177. - PubMed
-
- Ramalingam SS, Owonikoko TK, Khuri FR (2011) Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 61: 91–112. - PubMed
-
- Vallieres E, Peters S, Van Houtte P, Dalal P, Lim E (2011) Therapeutic advances in non-small cell lung cancer. Thorax. - PubMed
-
- Reungwetwattana T, Weroha SJ, Molina JR (2011) Oncogenic Pathways, Molecularly Targeted Therapies, and Highlighted Clinical Trials in Non-Small-Cell Lung Cancer (NSCLC). Clin Lung Cancer. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

