Leishmania donovani develops resistance to drug combinations
- PMID: 23285310
- PMCID: PMC3527373
- DOI: 10.1371/journal.pntd.0001974
Leishmania donovani develops resistance to drug combinations
Abstract
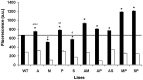
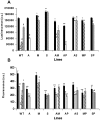
Drug combinations for the treatment of leishmaniasis represent a promising and challenging chemotherapeutic strategy that has recently been implemented in different endemic areas. However, the vast majority of studies undertaken to date have ignored the potential risk that Leishmania parasites could develop resistance to the different drugs used in such combinations. As a result, this study was designed to elucidate the ability of Leishmania donovani to develop experimental resistance to anti-leishmanial drug combinations. The induction of resistance to amphotericin B/miltefosine, amphotericin B/paromomycin, amphotericin B/Sb(III), miltefosine/paromomycin, and Sb(III)/paromomycin was determined using a step-wise adaptation process to increasing drug concentrations. Intracellular amastigotes resistant to these drug combinations were obtained from resistant L. donovani promastigote forms, and the thiol and ATP levels and the mitochondrial membrane potential of the resistant lines were analysed. Resistance to drug combinations was obtained after 10 weeks and remained in the intracellular amastigotes. Additionally, this resistance proved to be unstable. More importantly, we observed that promastigotes/amastigotes resistant to one drug combination showed a marked cross-resistant profile to other anti-leishmanial drugs. Additionally, the thiol levels increased in resistant lines that remained protected against the drug-induced loss of ATP and mitochondrial membrane potential. We have therefore demonstrated that different resistance patterns can be obtained in L. donovani depending upon the drug combinations used. Resistance to the combinations miltefosine/paromomycin and Sb(III)/paromomycin is easily obtained experimentally. These results have been validated in intracellular amastigotes, and have important relevance for ensuring the long-term efficacy of drug combinations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bryceson A (2001) A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health 6: 928–934. - PubMed
-
- Chunge CN, Owate J, Pamba HO, Donno L (1990) Treatment of visceral leishmaniasis in Kenya by aminosidine alone or combined with sodium stibogluconate. Trans R Soc Trop Med Hyg 84: 221–225. - PubMed
-
- Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen JA, et al. (2005) Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980–2004. Lancet Infect Dis 5: 763–774. - PubMed
-
- Sundar S, Rai M, Chakravarty J, Agarwal D, Agarwal N, et al. (2008) New treatment approach in Indian visceral leishmaniasis: single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin Infect Dis 47: 1000–1006. - PubMed

