Structural and functional control of the eukaryotic mRNA decapping machinery
- PMID: 23287066
- PMCID: PMC3660425
- DOI: 10.1016/j.bbagrm.2012.12.006
Structural and functional control of the eukaryotic mRNA decapping machinery
Abstract
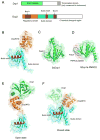
The regulation of mRNA degradation is critical for proper gene expression. Many major pathways for mRNA decay involve the removal of the 5' 7-methyl guanosine (m(7)G) cap in the cytoplasm to allow for 5'-to-3' exonucleolytic decay. The most well studied and conserved eukaryotic decapping enzyme is Dcp2, and its function is aided by co-factors and decapping enhancers. A subset of these factors can act to enhance the catalytic activity of Dcp2, while others might stimulate the remodeling of proteins bound to the mRNA substrate that may otherwise inhibit decapping. Structural studies have provided major insights into the mechanisms by which Dcp2 and decapping co-factors activate decapping. Additional mRNA decay factors can function by recruiting components of the decapping machinery to target mRNAs. mRNA decay factors, decapping factors, and mRNA substrates can be found in cytoplasmic foci named P bodies that are conserved in eukaryotes, though their function remains unknown. In addition to Dcp2, other decapping enzymes have been identified, which may serve to supplement the function of Dcp2 or act in independent decay or quality control pathways. This article is part of a Special Issue entitled: RNA Decay mechanisms.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures



References
-
- Lewis JD, Izaurralde E. The role of the cap structure in RNA processing and nuclear export. Eur J Biochem. 1997;247:461–9. - PubMed
-
- McKendrick L, Pain VM, Morley SJ. Translation initiation factor 4E. Int J Biochem Cell Biol. 1999;31:31–5. - PubMed
-
- Stevens A. An exoribonuclease from Saccharomyces cerevisiae: effect of modifications of 5′ end groups on the hydrolysis of substrates to 5′ mononucleotides. Biochem Biophys Res Commun. 1978;81:656–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

