ATF6 [corrected] and thrombospondin 4: the dynamic duo of the adaptive endoplasmic reticulum stress response
- PMID: 23287452
- PMCID: PMC3711712
- DOI: 10.1161/CIRCRESAHA.112.280560
ATF6 [corrected] and thrombospondin 4: the dynamic duo of the adaptive endoplasmic reticulum stress response
Erratum in
- Circ Res. 2013 Feb 1;112(3):e31
Abstract
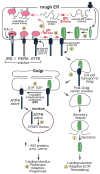
Thrombospondins are secreted, extracellular matrix (ECM) proteins that are upregulated in the heart and other tissues in response to ischemic injury and myocardial stress. Roles for thrombospondins after they are secreted have been examined in a variety of disease models, including myocardial pathology. However, a recent study published in the journal Cell by Lynch et al shifts this paradigm by focusing on roles for intracellular thrombospondins; these authors showed that thrombospondin-4 (Thbs4) can function from within cells to protect the heart by enhancing adaptive aspects of the endoplasmic reticulum (ER) stress response that are mediated by activating transcription factor 6, ATF6. Although this study was carried out in the cardiac context, the results add to our understanding of protein folding and quality control in all tissues. Moreover, the findings underscore the potential widespread therapeutic benefit of enhancing adaptive responses that are regulated by ATF6.
Figures
Comment on
-
A thrombospondin-dependent pathway for a protective ER stress response.Cell. 2012 Jun 8;149(6):1257-68. doi: 10.1016/j.cell.2012.03.050. Cell. 2012. PMID: 22682248 Free PMC article.
Similar articles
-
Sarco/endoplasmic reticulum calcium ATPase-2 expression is regulated by ATF6 during the endoplasmic reticulum stress response: intracellular signaling of calcium stress in a cardiac myocyte model system.J Biol Chem. 2001 Dec 21;276(51):48309-17. doi: 10.1074/jbc.M107146200. Epub 2001 Oct 10. J Biol Chem. 2001. PMID: 11595740
-
Establishment and validation of an endoplasmic reticulum stress reporter to monitor zebrafish ATF6 activity in development and disease.Dis Model Mech. 2020 Jan 28;13(1):dmm041426. doi: 10.1242/dmm.041426. Dis Model Mech. 2020. PMID: 31852729 Free PMC article.
-
Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response.J Biol Chem. 2000 Sep 1;275(35):27013-20. doi: 10.1074/jbc.M003322200. J Biol Chem. 2000. PMID: 10856300
-
[Endoplasmic reticulum-mediated integrated stress response].Sheng Li Ke Xue Jin Zhan. 2013 Aug;44(4):241-6. Sheng Li Ke Xue Jin Zhan. 2013. PMID: 24228513 Review. Chinese.
-
[Endoplasmic reticulum stress and angiogenesis].Fiziol Zh (1994). 2013;59(4):93-106. Fiziol Zh (1994). 2013. PMID: 24175483 Review. Ukrainian.
Cited by
-
Dissection of Thrombospondin-4 Domains Involved in Intracellular Adaptive Endoplasmic Reticulum Stress-Responsive Signaling.Mol Cell Biol. 2015 Oct 12;36(1):2-12. doi: 10.1128/MCB.00607-15. Print 2016 Jan 1. Mol Cell Biol. 2015. PMID: 26459760 Free PMC article.
-
ER Protein Quality Control and the Unfolded Protein Response in the Heart.Curr Top Microbiol Immunol. 2018;414:193-213. doi: 10.1007/82_2017_54. Curr Top Microbiol Immunol. 2018. PMID: 29026925 Free PMC article. Review.
-
Broken heart: A matter of the endoplasmic reticulum stress bad management?World J Cardiol. 2019 Jun 26;11(6):159-170. doi: 10.4330/wjc.v11.i6.159. World J Cardiol. 2019. PMID: 31367278 Free PMC article. Review.
-
The molecular mechanism of thrombospondin family members in cardiovascular diseases.Front Cardiovasc Med. 2024 Mar 7;11:1337586. doi: 10.3389/fcvm.2024.1337586. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38516004 Free PMC article. Review.
-
Modulation of cardiac fibrosis by Krüppel-like factor 6 through transcriptional control of thrombospondin 4 in cardiomyocytes.Cardiovasc Res. 2015 Sep 1;107(4):420-30. doi: 10.1093/cvr/cvv155. Epub 2015 May 17. Cardiovasc Res. 2015. PMID: 25987545 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

