Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles
- PMID: 23287580
- PMCID: PMC3655789
- DOI: 10.4161/cam.23130
Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles
Abstract
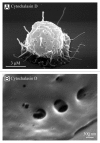
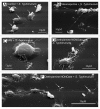
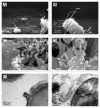
In this review, we summarized data on the formation and structure of the long and highly adhesive membrane tubulovesicular extensions (TVEs, membrane tethers or cytonemes) observed in human neutrophils and other mammalian cells, protozoan parasites and bacteria. We determined that TVEs are membrane protrusions characterized by a uniform diameter (130-250 nm for eukaryotic cells and 60-90 nm for bacteria) along the entire length, an outstanding length and high rate of development and a high degree of flexibility and capacity for shedding from the cells. This review represents TVEs as protrusions of the cellular secretory process, serving as intercellular adhesive organelles in eukaryotic cells and bacteria. An analysis of the physical and chemical approaches to induce TVEs formation revealed that disrupting the actin cytoskeleton and inhibiting glucose metabolism or vacuolar-type ATPase induces TVE formation in eukaryotic cells. Nitric oxide is represented as a physiological regulator of TVE formation.
Keywords: bacteria; cell secretion; cell-cell communications; cytonemes; eukaryotic cells; exocytotic carriers; host-pathogen interactions; membrane tethers; membrane tubulovesicular extensions (TVEs); protozoan parasites.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
