Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression
- PMID: 23288157
- PMCID: PMC4158029
- DOI: 10.1161/ATVBAHA.112.300329
Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression
Abstract
Objective: Macrophages are critical contributors to abdominal aortic aneurysm (AAA) disease. We examined the ability of MKEY, a peptide inhibitor of CXCL4-CCL5 interaction, to influence AAA progression in murine models.
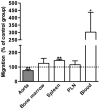
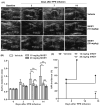
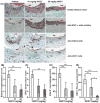
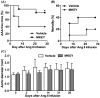
Approach and results: AAAs were created in 10-week-old male C57BL/6J mice by transient infrarenal aortic porcine pancreatic elastase infusion. Mice were treated with MKEY via intravenous injection either (1) before porcine pancreatic elastase infusion or (2) after aneurysm initiation. Immunostaining demonstrated CCL5 and CCR5 expression on aneurysmal aortae and mural monocytes/macrophages, respectively. MKEY treatment partially inhibited migration of adaptively transferred leukocytes into aneurysmal aortae in recipient mice. Although all vehicle-pretreated mice developed AAAs, aneurysms formed in only 60% (3/5) and 14% (1/7) of mice pretreated with MKEY at 10 and 20 mg/kg, respectively. MKEY pretreatment reduced aortic diameter enlargement, preserved medial elastin fibers and smooth muscle cells, and attenuated mural macrophage infiltration, angiogenesis, and aortic metalloproteinase 2 and 9 expression after porcine pancreatic elastase infusion. MKEY initiated after porcine pancreatic elastase infusion also stabilized or reduced enlargement of existing AAAs. Finally, MKEY treatment was effective in limiting AAA formation after angiotensin II infusion in apolipoprotein E-deficient mice.
Conclusions: MKEY suppresses AAA formation and progression in 2 complementary experimental models. Peptide inhibition of CXCL4-CCL5 interactions may represent a viable translational strategy to limit progression of human AAA disease.
Figures


Comment in
-
Targeting chemokines in aortic aneurysm: could this be key to a novel therapy for a common problem?Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):670-2. doi: 10.1161/ATVBAHA.112.301004. Arterioscler Thromb Vasc Biol. 2013. PMID: 23486768 No abstract available.
References
-
- Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Review of current theories for abdominal aortic aneurysm pathogenesis. Vascular. 2009;17:253–263. - PubMed
-
- Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, Gerszten RE. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol. 2007;179:691–697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

