ISPD gene mutations are a common cause of congenital and limb-girdle muscular dystrophies
- PMID: 23288328
- PMCID: PMC3562076
- DOI: 10.1093/brain/aws312
ISPD gene mutations are a common cause of congenital and limb-girdle muscular dystrophies
Abstract
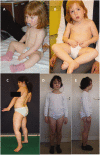
Dystroglycanopathies are a clinically and genetically diverse group of recessively inherited conditions ranging from the most severe of the congenital muscular dystrophies, Walker-Warburg syndrome, to mild forms of adult-onset limb-girdle muscular dystrophy. Their hallmark is a reduction in the functional glycosylation of α-dystroglycan, which can be detected in muscle biopsies. An important part of this glycosylation is a unique O-mannosylation, essential for the interaction of α-dystroglycan with extracellular matrix proteins such as laminin-α2. Mutations in eight genes coding for proteins in the glycosylation pathway are responsible for ∼50% of dystroglycanopathy cases. Despite multiple efforts using traditional positional cloning, the causative genes for unsolved dystroglycanopathy cases have escaped discovery for several years. In a recent collaborative study, we discovered that loss-of-function recessive mutations in a novel gene, called isoprenoid synthase domain containing (ISPD), are a relatively common cause of Walker-Warburg syndrome. In this article, we report the involvement of the ISPD gene in milder dystroglycanopathy phenotypes ranging from congenital muscular dystrophy to limb-girdle muscular dystrophy and identified allelic ISPD variants in nine cases belonging to seven families. In two ambulant cases, there was evidence of structural brain involvement, whereas in seven, the clinical manifestation was restricted to a dystrophic skeletal muscle phenotype. Although the function of ISPD in mammals is not yet known, mutations in this gene clearly lead to a reduction in the functional glycosylation of α-dystroglycan, which not only causes the severe Walker-Warburg syndrome but is also a common cause of the milder forms of dystroglycanopathy.
Figures




References
-
- Bonnemann CG, Finkel RS. Sarcolemmal proteins and the spectrum of limb-girdle muscular dystrophies. Semin Pediatr Neurol. 2002;9:81–99. - PubMed
-
- Bourteel H, Vermersch P, Cuisset JM, Maurage CA, Laforet P, Richard P, et al. Clinical and mutational spectrum of limb-girdle muscular dystrophy type 2I in 11 French patients. Journal of neurology, neurosurgery, and psychiatry. 2009;80:1405–8. - PubMed
-
- Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16:29R–37R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

