Diffusion tensor imaging of normal brain development
- PMID: 23288475
- PMCID: PMC3703661
- DOI: 10.1007/s00247-012-2496-x
Diffusion tensor imaging of normal brain development
Abstract
Diffusion tensor imaging (DTI) is an MRI technique that can measure the macroscopic structural organization in brain tissues. DTI has been shown to provide information complementary to relaxation-based MRI about the changes in the brain's microstructure. In the pediatric population, DTI enables quantitative observation of the maturation process of white matter structures. Its ability to delineate various brain structures during developmental stages makes it an effective tool with which to characterize both the normal and abnormal anatomy of the developing brain. This review will highlight the advantages, as well as the common technical pitfalls of pediatric DTI. In addition, image quantification strategies for various DTI-derived parameters and the normal brain developmental changes associated with these parameters are discussed.
Figures
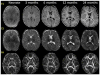
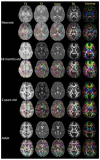


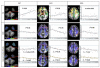
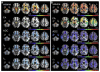
References
-
- Barkovich AJ, Raybaud C. Pediatric neuroimaging. 5th edn. Lippincott Williams & Wilkins; Philadelphia: 2012.
-
- van der Knaap MS, Valk J. Magnetic resonance of myelination and myelin disorders. 3rd edn. Springer; Berlin: 2005.
-
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell; Oxford: 1967.
-
- Ballesteros MC, Hansen PE, Solla K. MR imaging of the developing human brain. Part 2. Postnatal development. Radiographics. 1993;13:611–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

