Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide
- PMID: 23288535
- PMCID: PMC4365795
- DOI: 10.1126/science.1230631
Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide
Abstract
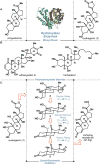
Here, we report on a scalable route to the polyhydroxylated steroid ouabagenin with an unusual take on the age-old practice of steroid semisynthesis. The incorporation of both redox and stereochemical relays during the design of this synthesis resulted in efficient access to more than 500 milligrams of a key precursor toward ouabagenin-and ultimately ouabagenin itself-and the discovery of innovative methods for carbon-hydrogen (C-H) and carbon-carbon activation and carbon-oxygen bond homolysis. Given the medicinal relevance of the cardenolides in the treatment of congestive heart failure, a variety of ouabagenin analogs could potentially be generated from the key intermediate as a means of addressing the narrow therapeutic index of these molecules. This synthesis also showcases an approach to bypass the historically challenging problem of selective C-H oxidation of saturated carbon centers in a controlled fashion.
Figures


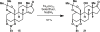
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

